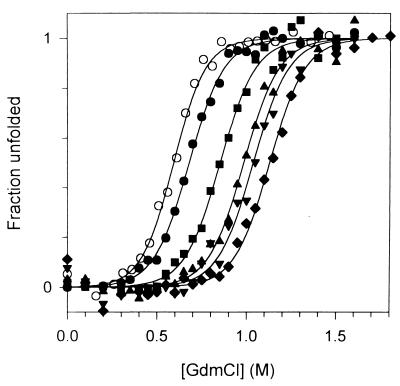
Figure 2.
GdmCl-induced equilibrium unfolding transition of mutant α1AT. Equilibrium unfolding of α1AT was monitored by fluorescence spectroscopy with excitation at 280 nm and emission at 360 nm. Native α1AT was diluted into an appropriate GdmCl solution and incubated at 25°C for 4 h. The buffer was 10 mM sodium phosphate/50 mM NaCl/1 mM EDTA, pH 6.5, and the final protein concentration was 5 μg/ml. ○, wild type; ●, G117A; ■, G117V; ▴, G117L; ▾, G117I; ♦, G117F.