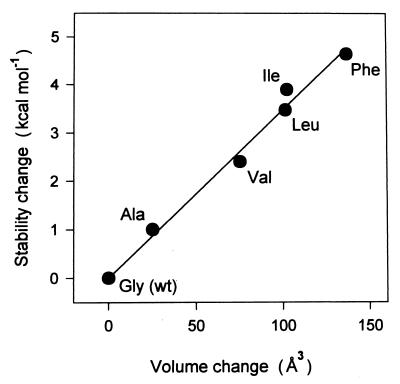
Figure 3.
Effect of amino acid substitutions for Gly-117 on the stability of α1AT. Changes in free energy of stabilization (ΔΔG) as a function of the increase in van der Waals volume of the side chain are plotted. The difference in stabilization between the mutant and the wild-type α1AT was calculated from ΔΔG = ΔCm × 7.9 kcal⋅mol−1⋅M−1 (average m value: denaturant-dependent free energy change) where ΔCm is the difference between the concentration at the transition midpoint of the wild-type and that of mutant α1AT measured by equilibrium unfolding experiments as in Fig. 2. Each label is the three-letter code for the amino acid substituted at the 117 site, and wt indicates wild-type protein. The line through the data points was obtained by least-squares fit.