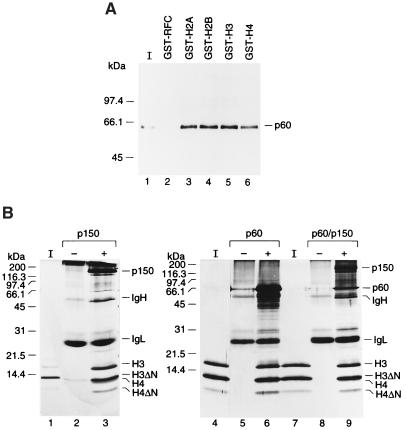
Figure 5.
The N termini of H3.H4 are not required for association of p150 or p60 with H3.H4. (A) Binding of p60 to histones. A total of 50 pmol of recombinant p60 was incubated with excess (≈5 μg) GST-histones or GST-RFC (37-kDa subunit of human RFC) and pulled down with glutathione beads. Precipitated proteins were loaded on an 18% polyacrylamide gel and analyzed by Western blotting with anti-p60 monoclonal antibody. 25% of input histones (I) were loaded in lanes 1. (B) Competitive binding assay. A total of 50 pmol of wild-type or mutant (H3.H4)2 tetramers were immunoprecipitated by anti-CAF-1 antibodies (anti-p150 antibody for lanes 2, 3, 8, and 9 or anti-p60 antibody for lanes 5 and 6), either in the presence or absence of recombinant p150, p150/p60 (25 pmol), or p60 (50 pmol), as indicated by + or −. Precipitated proteins were resolved in an 18% polyacrylamide gel and silver stained. 12.5% of input histones (I) were loaded in lane 1. p150, p60, and p48, Ig heavy chain (IgH) and light chain (IgL), and individual histone H3 and H4 are indicated.