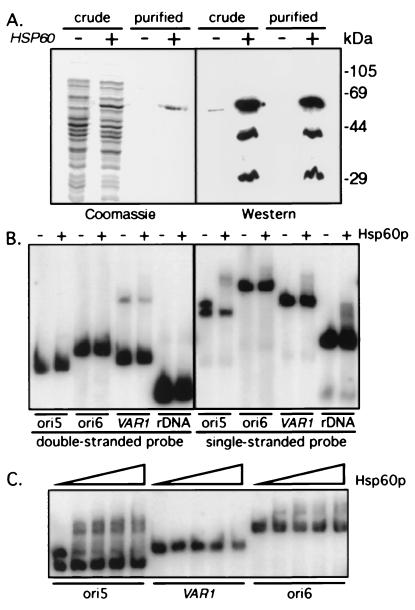
Figure 4.
Hsp60p-6His is a sequence-specific ssDNA binding protein. (A) Full-length HSP60 cloned into pET-24d (+) or vector alone (−) was induced in E. coli. Proteins present in the soluble extract (crude) or after Ni2+ column purification (purified) were fractionated through 10% polyacrylamide gels and either stained with Coomassie blue or subjected to Western blot analysis with anti-Hsp60p serum. Two polypeptides (44 and 29 kDa) copurified with Hsp60p-6His and presumably represent proteolytic products. (B) dsDNA or ssDNA probes (ori5, ori6, VAR1, and rDNA) were incubated in the presence (+) or absence (−) of 15 ng Hsp60p-6His and the protein–DNA complexes were separated from free DNA by electrophoresis through 3.5% polyacrylamide gels. Hsp60p-6His bound preferentially to one strand of ori5, in contrast to the absence of binding to the ds substrates and to the much reduced binding to the other ss substrates. (C) ssDNAs were prepared by boiling and quick cooling on ice and increasing concentrations of Hsp60p-6His (1.5, 3, 6, 12 ng) were added to the indicated probes, and protein–DNA complexes fractionated as above.