Fig. 1.
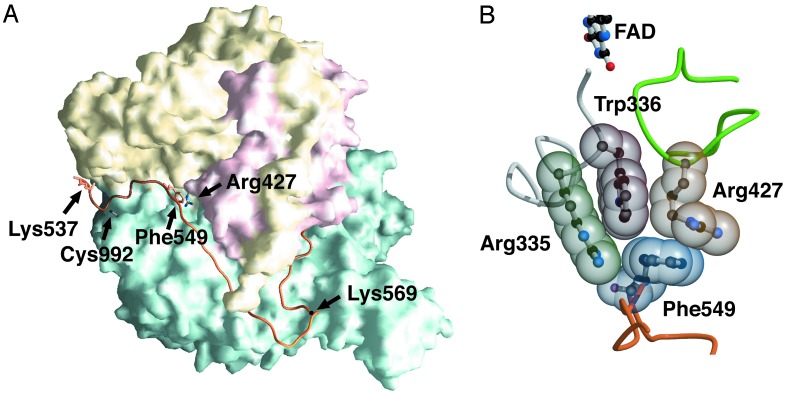
(A) Molecular surface of the XDH monomer divided into three major domains. The domains are iron/sulfur-center (residues 3–165; red), FAD (residues 226–531; yellow), and Mo-pt (residues 590–1,331; blue). The linker connecting the FAD domain with the Mo-pt domain (residues 537–589) is shown in orange. The positions of Cys-992 and Lys-537, the one still visible in the electron density map and closest to Cys-535, are shown as a stick model. The positions of Lys-551 and Lys-569, responsible for proteolysis, are also indicated. The positions of Phe-549, which is located on the linker connecting the Mo and FAD domains, and Arg-427, which is located on the sliding loop, are represented as stick models. (B) Space-filling model of the important cluster residues in the XDH form that are involved in the XDH/XO conversion.