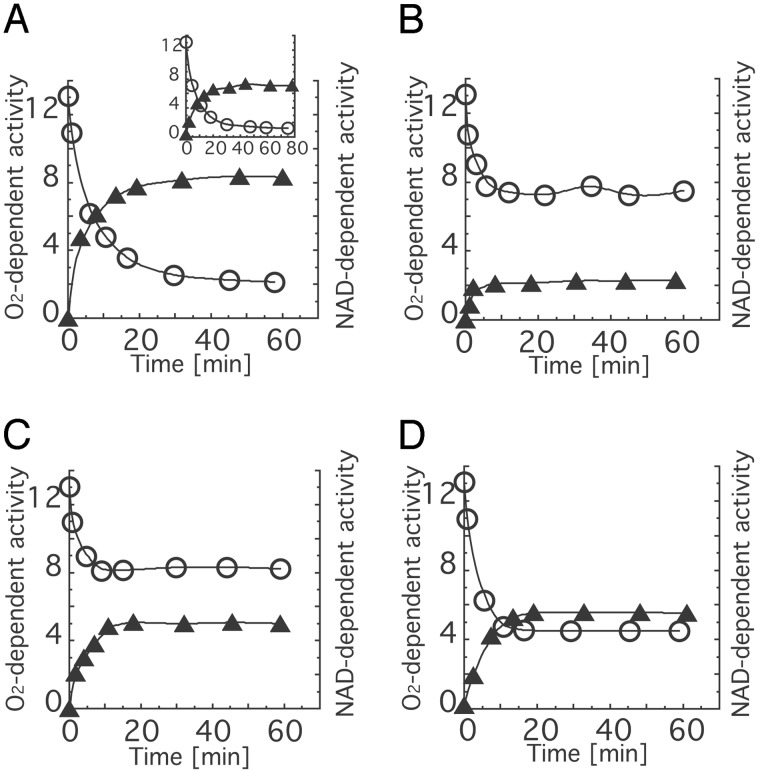
Fig. 4.
Time course of XDH/XO conversion of wild-type and mutant enzymes by incubation with dithiothreitol. Each protein was incubated with 5.0 mM DTT at pH 8.5 and 25°C. During the incubation, aliquots were withdrawn from the mixture to measure the enzyme activities. The activities were corrected for the measured value of AFR assuming that the AFR of fully active enzyme in XO and the freshly purified mutant enzymes was 200 (28). O2-dependent activity and NAD+-dependent activity are expressed as rate of urate formation [mol/mol per sec] and rate of NADH formation [mol/mol per sec], respectively. Open circles, O2-dependent activities; filled triangle, NAD+-dependent activities. Values for recombinant wild-type enzyme (A), bovine milk XOR (Insert), the W336A mutant (B), the R427Q mutant (C), and the R335A mutant (D) are shown.