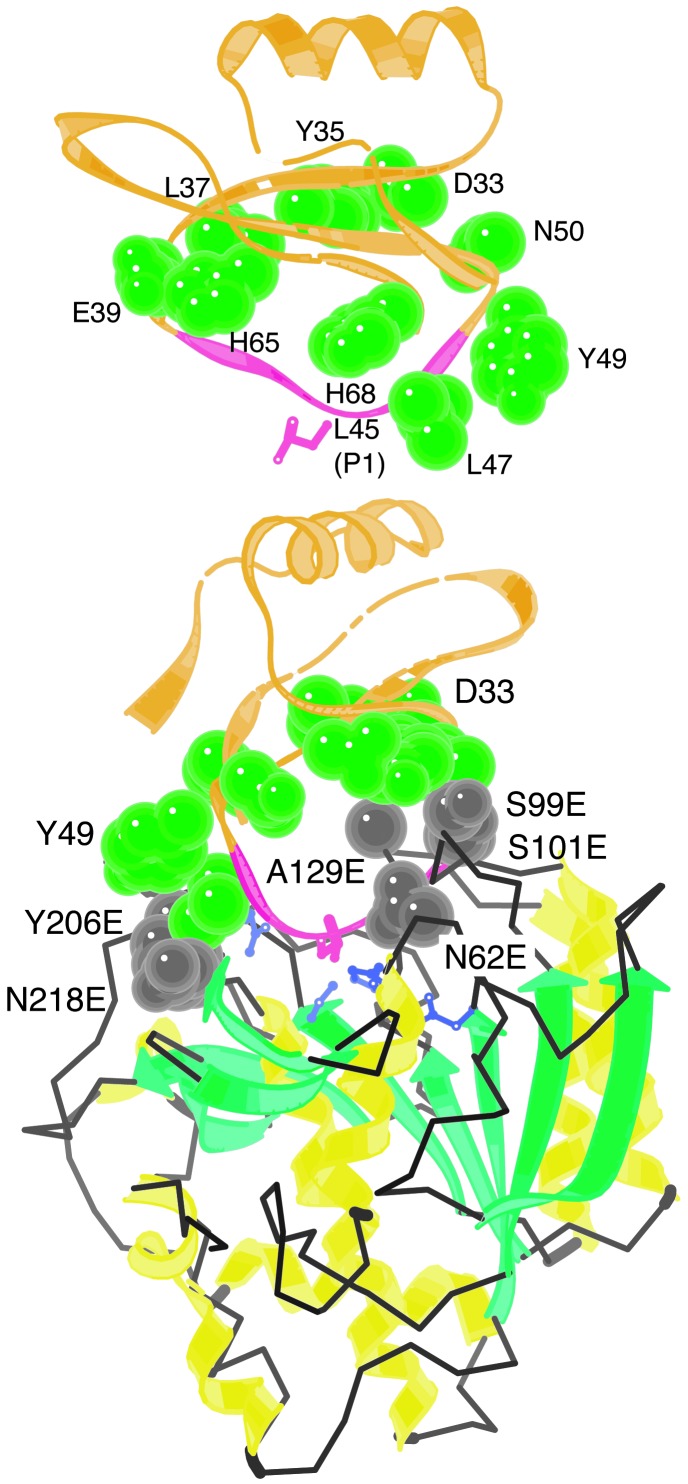
Fig. 2.
Eglin c residues with the potential to make adventitious contacts with Kex2/furin family enzymes. (Upper) Structure of eglin c alone, from crystal structure of a complex of eglin c with subtilisin Carlsberg. (Lower) Crystal structure of the complex (coordinates from 1cse.pdb, ref. 15). Structure was drawn with kinemage (28). Ten eglin c residues (exclusive of P4-P′1) whose side chains come within 7 Å of the subtilisin surface and that were mutagenized in this study are shown in green. Interacting enzyme residues are shown in gray and are indicated by residue numbers followed by E. P1 Leu-45 of eglin c is shown in pink and catalytic His (His-64) is shown in purple.