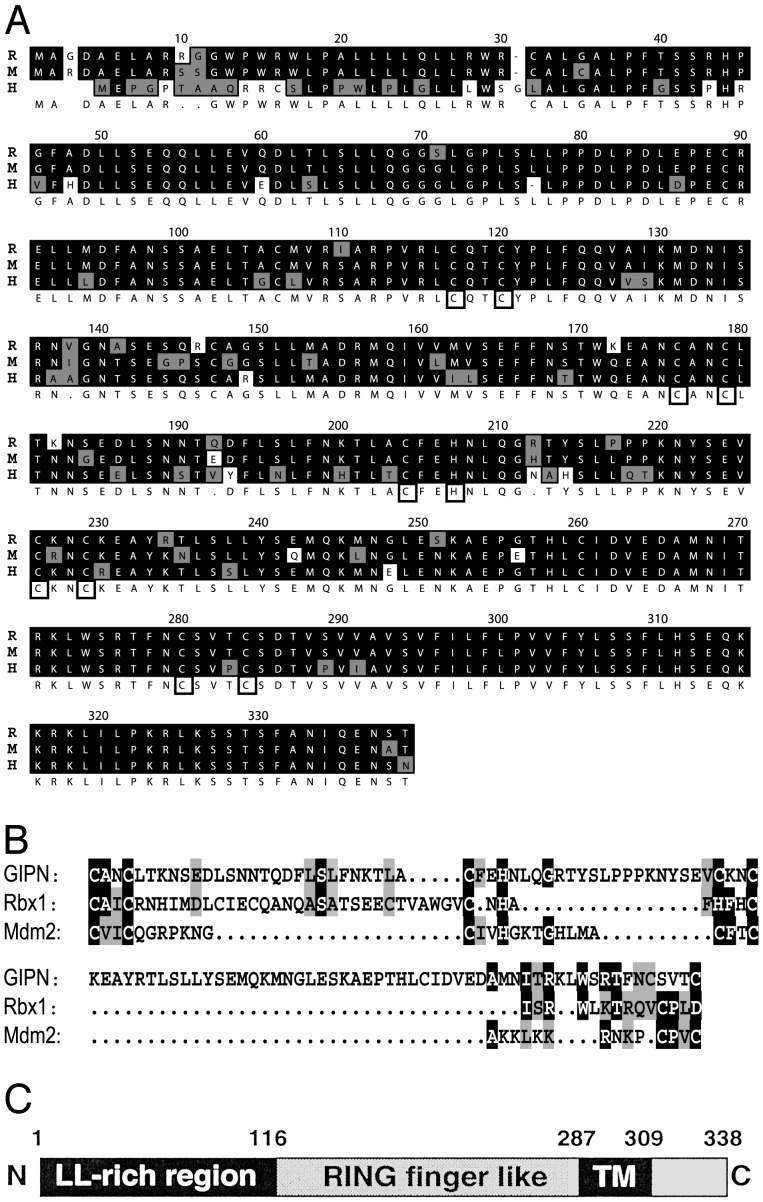
Fig. 1.
(A) Alignment of rat (R), mouse (M), and human (H) GIPN proteins. Identical residues are shaded in black, and conserved residues are shaded in gray; cysteine and histidine pairs are boxed. (B) The RING finger-like domain of GIPN (amino acids 175–283) was aligned with the RING finger of Rbx1(amino acids 42–97) and Mdm2 (amino acids 438–478). (C) GIPN is predicted to be an integral membrane protein with a large RING finger-like cytoplasmic domain, a putative transmembrane domain (amino acids 287–309), and a short (30-aa) C terminus. The N terminus of GIPN (amino acids 1–110) contains several dileucine motifs (LL-rich region).