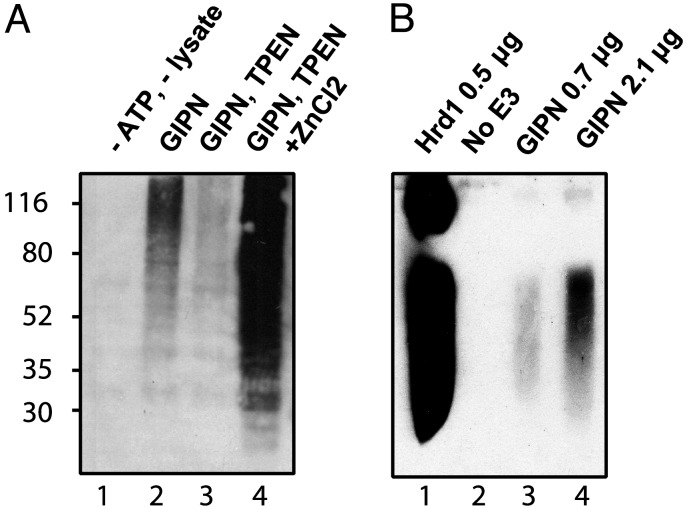
Fig. 7.
GIPN has in vitro Zn2+-sensitive ubiquitination activity. (A) Autopolyubiquitination of GIPN is Zn2+-dependent. GST-GIPN bound to glutathione-agarose beads was incubated with ubiquitin and MG-132 in the presence or absence of ATP and lysate. Proteins were immunoblotted with anti-ubiquitin. The broad band (lane 2) indicates polyubiquitination. When lysates are preincubated with the Zn2+ chelator TPEN, polyubiquitination is significantly diminished (lane 3). When a 10-fold concentration of ZnCl2 (relative to TPEN) is added, polyubiquitination is greatly enhanced (lane 4). No polyubiquitination is detected in the absence of ATP and lysate (lane 1). (B) GIPN undergoes autoubiquitination in the presence of E1 and E2. Purified recombinant E1, E2 (Ubc4), and Hrd1 or GIPN were incubated with ubiquitin and ATP. Proteins were immunoblotted with anti-ubiquitin. GIPN is able to autoubiquitinate itself in a concentration-dependent manner (lanes 3 and 4). In the absence of E3 (lane 2), no signal can be detected. Hrd1 (lane 1) was used as a positive control.