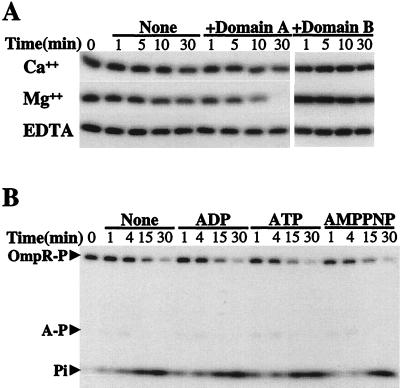
Figure 1.
Phosphatase activity of domain A. (A) Metal ion-dependent phosphatase activity of domains A and B. Purified OmpR-P (1.67 μM) was incubated with or without 3.34 μM domain A or B protein in phosphatase reaction buffer (50 mM Tris⋅HCl, pH 8.0/50 mM KCl/5% glycerol) containing 5 mM CaCl2 (Top), 5 mM MgCl2 (Middle), or 5 mM EDTA (Bottom) at room temperature. Aliquots were removed at the indicated time points and the reactions were stopped by 5× SDS gel loading buffer. The reaction mixtures were then analyzed by SDS/PAGE followed by autoradiography as well as PhosphorImager quantification. (B) Effect of cofactors on the phosphatase activity of domain A. The phosphatase reactions were carried out in Mg2+-containing buffer described for A in the presence of 1 mM ADP, ATP, or AMPPNP. The positions of phosphorylated domain A and Pi are indicated by arrows.