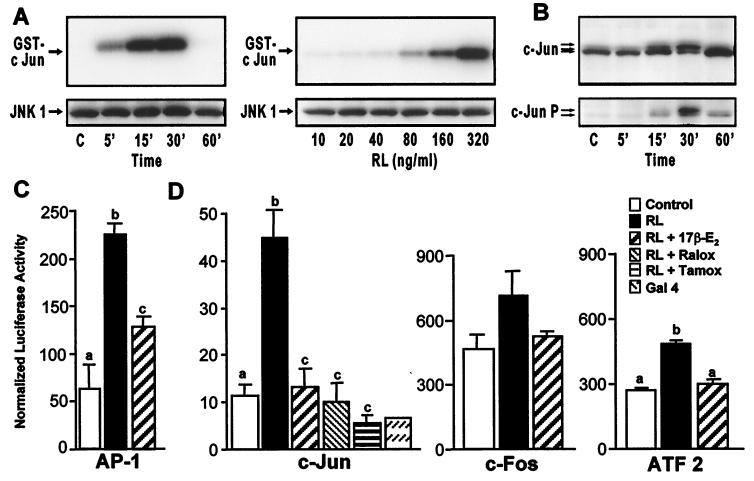
Figure 4.
Estrogen suppresses RANKL-induced activation of c-Jun and AP-1-mediated transcription in RAW264.7 cells. (A) Cells were treated for the indicated times with RANKL (80 ng/ml) or with the indicated concentrations of RANKL (RL) for 15 min and lysates examined for both JNK1 protein by Western blot and kinase activity (using GST-cJun as substrate). (B) Cells were treated for the indicated times with RANKL (80 ng/ml) and then subjected to Western blot analysis by using antibodies to c-Jun or phospho-c-Jun. Arrows indicate c-Jun and phospho-c-Jun forms. (C) Cells were transfected with a luciferase reporter plasmid (p36) containing three copies of an AP-1 response element. Cells were treated with vehicle or M-CSF and RANKL in the absence or presence of 17β-estradiol (10−8 M). Cells were harvested 24 h later and lysates assessed for luciferase and β-galactosidase activities and protein. Numbers represent the mean ± SE, n = 3 (a is significant vs. b and c; and b is significant vs. c at P ≤ 0.05). The results are representative of three independent experiments. (D) RAW264.7 cells were cotransfected with pFC2-luc and one of the following plasmids: pFA2-cJun (c-Jun), pFA-cFos (c-Fos), pFA-ATF2 (ATF2), or pFC-dbd (Gal4). Following transfection, cells were treated with RANKL in the presence of vehicle, 17β-estradiol (10−8 M), 4-hydroxytamoxifen (10−7 M), or raloxifene (10−7 M). Cells were harvested 24 h later and lysates assessed for luciferase activity, β-galactosidase activity, and protein. Numbers represent the mean ± SE, n = 3 (b is significant vs a and c at P ≤ 0.05). The results are representative of at least three independent experiments.