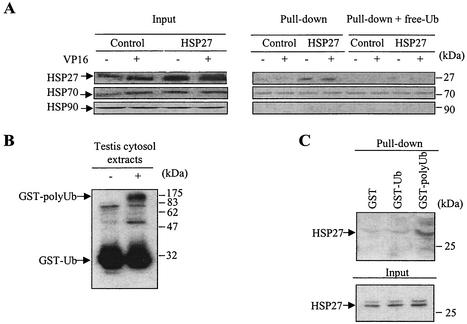
FIG. 3.
HSP27 associates with ubiquitin. (A) Cytoplasmic extracts obtained from control-transfected and HSP27-transfected U937 cells, either left untreated or treated with 100 μM etoposide (VP16) for 4 h, were incubated with a ubiquitin-agarose matrix. The presence of the indicated proteins in the input material was analyzed by Western blotting (left panels). Pull-down analysis of ubiquitin-agarose was performed in the absence or presence of 50 μg of free ubiquitin (free-Ub) before elution of bound proteins and Western blot analysis of HSPs (right panels). One representative experiment of three independent experiments is shown. (B) Western blot analysis of GST-monoubiquitin (GST-Ub) and GST-polyubiquitin (GST-polyUb) obtained in vitro as described in Material and Methods. (C) Cytosolic extracts from HSP27-overexpressing cells were incubated with GST alone, GST-Ub, or GST-polyUb. The presence of HSP27 in input material was checked by Western blotting after pull-down analysis and elution of bound proteins. One representative experiment of three experiments is shown.