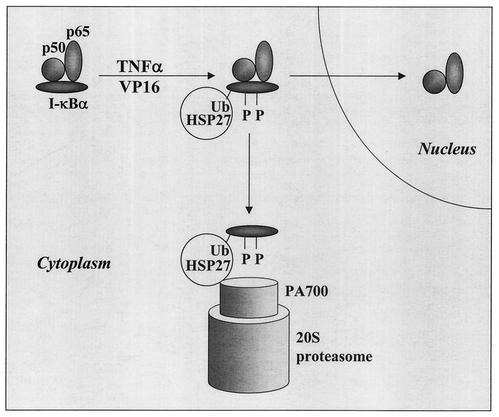
FIG. 9.
Hypothetical model for the influence of HSP27 on proteasome-mediated I-κBα degradation. In response to stimulation, I-κBα is phosphorylated and ubiquitinated (Ub), which could enhance its association with HSP27 and its targeting to the 26S proteasome, thus permitting the release of NF-κB dimers that translocate to the nucleus. When associated with ubiquitinated and phosphorylated I-κBα, HSP27 may favor its transfer, recognition, and/or unfolding by the 26S proteasome.