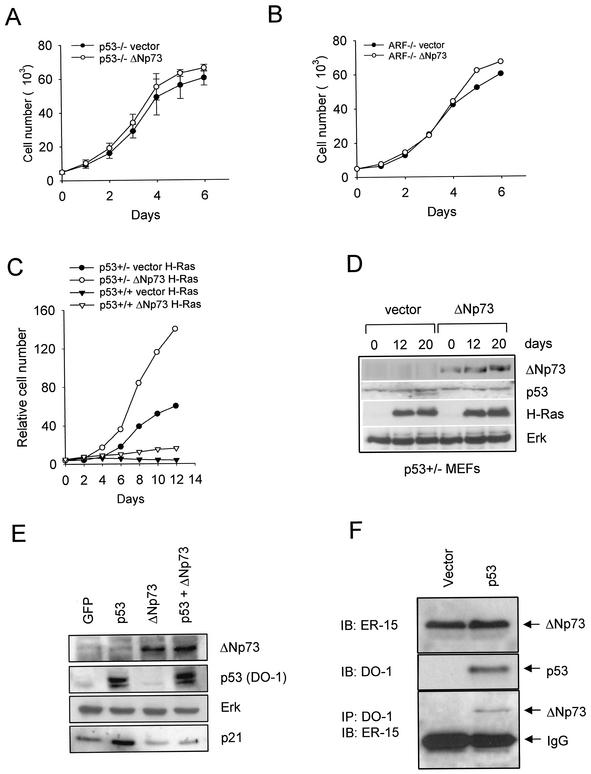
FIG. 8.
(A and B) In the absence of p53 or ARF, ΔNp73 has no effect on proliferation. The growth kinetics of p53−/− MEFs and p19ARF−/− MEFs transduced with a control empty vector (•) or ΔNp73-expressing vector (○) are shown. ΔNp73-expressing cells showed no proliferative advantage over vector control cells. (C) Compared to p53+/+ MEFs, p53+/− MEFs exhibit enhanced ΔNp73-mediated rescue from Ras-induced growth arrest. Growth curves of 129 MEFs of the indicated genotypes transduced with recombinant empty Rpuro vector or ΔNp73, followed by transduction with H-RasV12 4 days later, are shown. The growth advantage bestowed by ΔNp73 in p53+/+ MEFs was further increased in p53+/− MEFs. Note the compressed y-axis scale compared to Fig. 1A. (D) Expression of transduced ΔNp73 and H-RasV12 over time in p53+/− MEFs from panel C. Cells were selected in puromycin for 5 days. Lanes: 1, 2, and 3, vector+H-RasV12 0, 12, and 20 days, respectively, after Ras infection; 4, 5, and 6, ΔNp73+H-RasV12 at 0, 12, and 20 days, respectively, after Ras infection, which occurred 4 days after vector/ΔNp73 infection.Immunoblots were performed with the indicated antibodies. Endogenous Erk is used as loading control. (E) ΔNp73 inhibits the transactivation function of wild-type p53 in primary fibroblasts. 129 wild-type MEFs were infected with retroviruses encoding either GFP, human wild-type p53, ΔNp73, or both p53 and ΔNp73. After 48 h, cells were analyzed by immunoblotting for endogenous p21Waf1. Retroviral expression was confirmed as indicated. Endogenous Erk was used as loading control. (F) Physical interaction between ΔNp73α and wild-type p53 protein in primary fibroblasts. At the bottom of the panel is shown the detection of a protein complex between ΔNp73α and wild-type p53 in an immortal ΔNp73-expressing MEF clone 48 h after infection with either a control or a p53-expressing retrovirus. Immunoprecipitations of equal amounts of total protein (2 mg each) with a mixture of the anti-p53 antibodies DO-1 and 1801 covalently coupled to agarose beads, followed by immunoblotting with anti-ΔNp73 antibody ER-15, were carried out. The immunoglobulin G light chain is indicated. At the top, immunoblots (50 μg of total protein) with the indicated antibodies are shown to confirm expression.