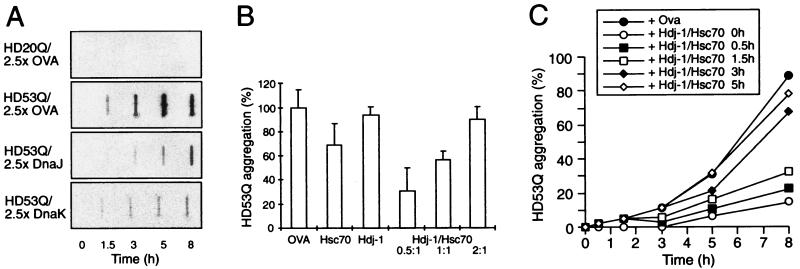
Figure 1.
Modulation of aggregation of HD exon 1 by Hsp70 and Hsp40 in vitro. (A) Time-dependent formation of SDS-insoluble aggregates of HD20Q and HD53Q (3 μM) in the presence and absence of ovalbumin (OVA) and DnaJ (Hsp40) or DnaK (Hsp70) of E. coli (7.5 μM each) in the absence of ATP as detected in filter-trap assays. Chaperones were added when aggregation was initiated by proteolytic cleavage of GST-HD fusion proteins. OVA served as a nonchaperone control protein. (B) Cooperation of mammalian Hdj-1 (Hsp40) and Hsc70 proteins in retarding SDS-insoluble HD53Q aggregation in the presence of ATP (2 mM). The ratio of Hdj-1 to Hsc70 was varied with Hsc70 and HD53Q at 3 μM. Amounts of aggregates after 8 h of incubation were quantified by Phosphorimager densitometry. Means and standard deviations of at least three independent experiments with similar results are shown. (C) Time course of formation of SDS-insoluble HD53Q aggregates with addition of Hsc70/Hdj-1/ATP (6 μM/3 μM/2 mM) occurring at different times after initiating aggregation by proteolytic cleavage of GST-HD53Q (3 μM).