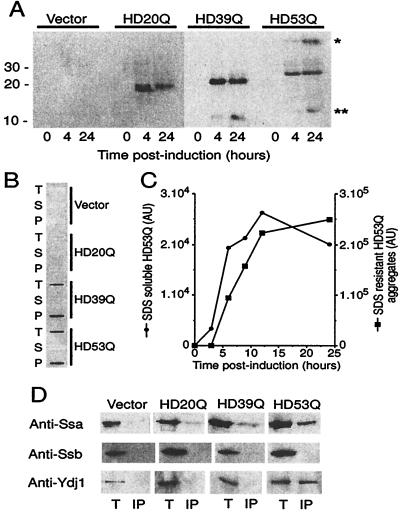
Figure 4.
Expression and aggregation of mutant HD exon 1 in yeast. (A) Expression of myc-tagged HD20Q, HD39Q, and HD53Q was analyzed over 24 h by SDS/PAGE and immunoblotting of cell lysates with anti-myc antibodies. (B) PolyQ length-dependent formation of SDS-insoluble HD aggregates. Equal protein amounts of cell lysates were analyzed in filter-trap assays after incubation in SDS/DTT at 95°C. T, total cell lysate; S and P, supernatant and pellet fractions after centrifugation of total cell lysate (20,000 × g, 10 min), respectively. (C) Kinetics of HD expression and aggregation. Expression of SDS-soluble HD53Q and formation of SDS-insoluble HD53Q aggregates were monitored by SDS/PAGE and filter-trap assay of total cell lysates as in A. Data are plotted in arbitrary densitometer units for equal amounts of cell protein analyzed. Densitometric units for SDS-soluble and -insoluble HD53Q are only approximately comparable. (D) Association of Hsp70 and Hsp40 with HD exon 1 in yeast. Immunoprecipitation of myc-tagged HD20Q, HD39Q, and HD53Q with anti-myc antibody from total cell lysates after 24 h of expression followed by immunoblotting of precipitates with anti-Ssa (yeast Hsp70), anti-Ssb (yeast Hsp70), and anti-Ydj1 (yeast Hsp40) antibodies. Immunoprecipitations were performed in the absence of ATP. T, total lysate; IP, immunoprecipitate. Protein amounts loaded in T correspond to approximately 10% of those loaded in IP. Anti-myc immunoprecipitation was ≈50% effective.