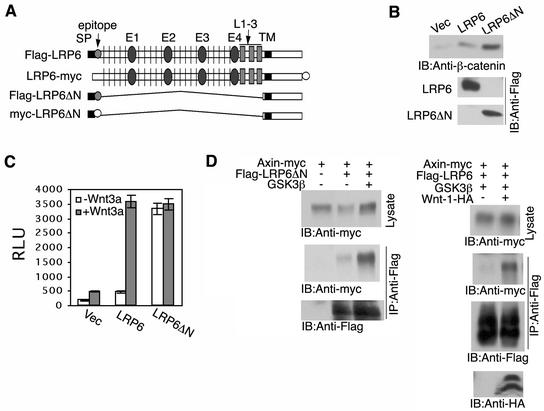
FIG. 1.
Extracellular domain deletion converts an inactive LRP6 to a constitutively active receptor. (A) Schematic representation of LRP6 and of extracellular domain-deleted LRP6 constructs (Flag or myc epitope tagged). Signal peptide (SP), EGF repeats (E1 to E4) separated by YWTD (vertical line) containing spacers, LDLR repeats 1 to 3 (L1-3), and transmembrane domain (TM) preceding the intracellular domain. (B) Comparison of β-catenin stabilization by LRP6ΔN and wild-type LRP6 receptors. At 48 h following transfection of 293T cells with 0.5 μg of either vector, LRP6, or LRP6ΔN, cell lysates were subjected to uncomplexed β-catenin analysis as described in Materials and Methods. IB, immunoblot. (C) Comparison of TCF signaling by LRP6ΔN and wild-type LRP6 receptors. 293T cells were transfected with 0.5 μg of vector (Vec), Flag-LRP6, or Flag-LRP6ΔN in the presence or absence of Wnt3a (0.1 μg) in 6-well dishes. In addition, the TCF reporter construct pGL3-OT was transfected at 1 μg, and the control luciferase construct, pRL-CMV, was transfected at 5 ng unless otherwise indicated. Relative luciferase units (RLU) were normalized against Renilla luciferase activity at 48 h after transfection. Results are expressed as the means ± standard deviations of two independent experiments performed in duplicate. (D) Ligand-dependent recruitment of Axin to the wild-type LRP6 receptor. Immunoprecipitation (IP) and immunoblot analyses were performed following transfection of 293T cells with 1 μg of each plasmid as indicated.