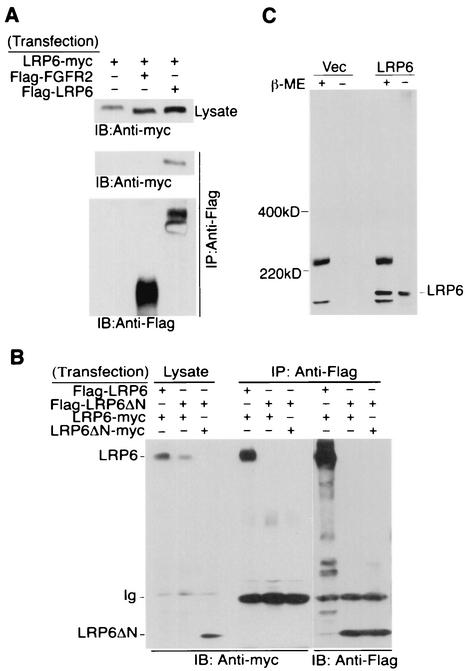
FIG. 2.
Oligomerization of the wild-type LRP6 receptors but not the mutant receptor lacking the extracellular domain. (A) Oligomerization of the wild-type LRP6 receptor. Forty-eight hours after transfection of 293T cells with 1 μg of each plasmid as indicated, immunoprecipitation (IP) and immunoblot (IB) analyses were performed from 1 mg of cell lysate. (B) Comparison of receptor complex formation by LRP6ΔN and LRP6. At 48 h following transfection of 293T cells with 2 μg each of Flag- or myc-tagged LRP6 constructs as indicated, immunoprecipitation with anti-Flag antibody was performed by using 1 mg of total cell lysate and was followed by immunoblotting with anti-myc antibody. (C) Comparison of LRP6 receptor migration under reducing and nonreducing conditions. Equal amounts of either vector or Flag-LRP6 expressing lysates were subjected to SDS-PAGE in the presence (+) or absence (−) of reducing agent β-mercaptoethanol (β-ME). Immunoblotting was performed with anti-Flag antibody. Ig, immunoglobulin.