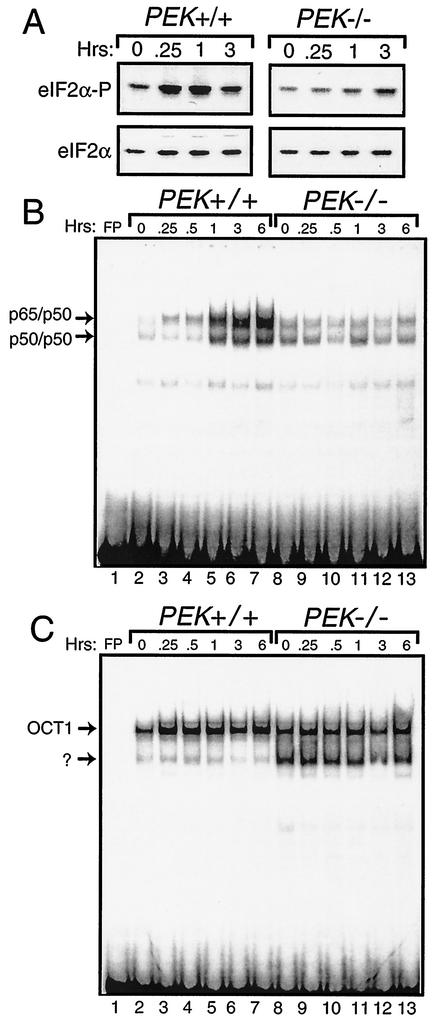
FIG. 1.
eIF2α kinase PEK is required for activation of NF-κB in response to ER stress. (A) PEK+/+ and PEK−/− MEF cells were exposed to thapsigargin for 0.25 to 3 h (as indicated) or in the absence of this ER stress agent (0 h). Phosphorylation of eIF2α was measured by immunoblot analysis by using polyclonal antibody specific to eIF2α phosphorylated at Ser-51 (eIF2α∼P). Levels of total eIF2α were assayed by using antibody that recognizes both phosphorylated and nonphosphorylated versions of the translation initiation factor. (i.e., eIF2α). Nuclear lysates were prepared from PEK+/+ (lanes 2 to 7) and PEK−/− (lanes 8 to 13) MEF cells treated with thapsigargin for the indicated times and incubated with radiolabeled DNA containing a NF-κB (B) or OCT1 (C) binding sites. Binding mixtures were separated by electrophoresis, and bound DNAs were visualized by autoradiography. Arrows indicate DNA complexed with p65/p50 or p50/p50 as defined in experiments shown in Fig. 2. OCT1 bound to DNA and an unknown protein complex are also indicated by arrows. Radiolabeled DNA at the bottom of panels B and C are unbound probe. Free probe (FP) indicates the radiolabeled NF-κB or OCT1 DNA fragments without nuclear lysate.