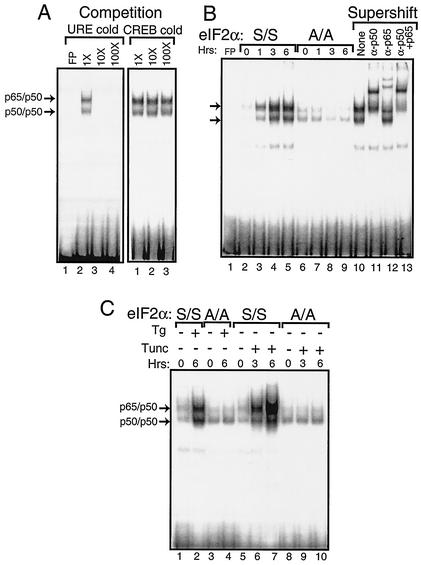
FIG. 2.
Phosphorylation of eIF2α at Ser-51 facilitates activation of NF-κB during ER stress. Nuclear lysates were prepared from MEF cells containing eIF2α with wild-type Ser-51 (S/S) or with alanine substituted for the eIF2α phosphorylation site (A/A) subjected to ER stress or to no stress. Equal amounts of nuclear lysate were used in each EMSA mixture containing radiolabeled DNA with an NF-κB binding site. Instances of DNA complexed with NF-κB dimers p65/p50 and p50/p50 (indicated by arrows) were visualized following autoradiography. (A) To determine the specificity for the NF-κB binding site, nonradiolabeled DNA containing the NF-κB site, URE (lanes 2 to 4), or the unrelated CREB DNA binding site (lanes 1 to 3) was added to EMSA binding mixtures containing nuclear lyates prepared from S/S MEF cells treated with thapsigargin for 6 h. Competition indicates that nonradiolabeled competitor DNA was added at a 1×, 10×, or 100× molar excess. Free probe (FP) indicates only radiolabeled NF-κB DNA fragments without nuclear lysate. Radiolabeled DNA at the bottom of panel is unbound probe. (B) Nuclear lysates were prepared from S/S (lanes 2 to 5) and A/A (lanes 6 to 9) MEF cells subjected to thapsigargin for between 0 and 6 h and assayed for NF-κB binding in the EMSA. In lanes 10 to 13, supershift indicates that polyclonal antibodies that specifically recognize p50 and/or p65 were added to the EMSA binding mixture. “None” indicates that no antibody was used in the assay (lane 10). (C) MEF cells were exposed to either thapsigargin (Tg) or tunicamycin (Tunc) as indicated by the “+” or “−” for the indicated number of hours. Nuclear lysates prepared from S/S and A/A MEF cells (as indicated) were analyzed for binding to the NF-κB probe in the EMSA.