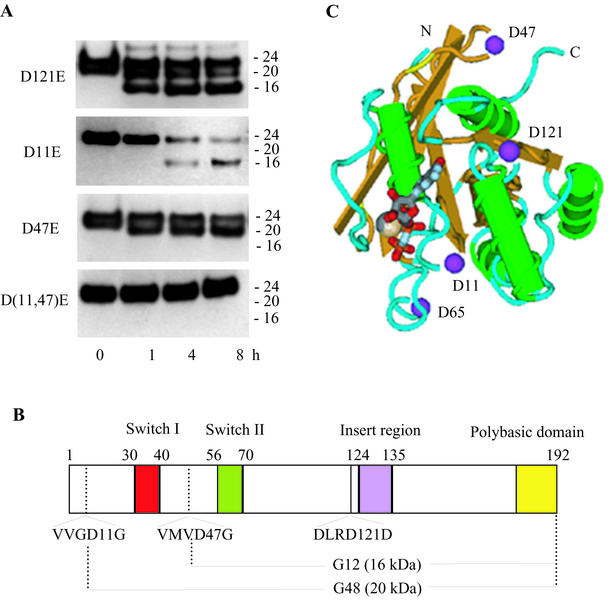
FIG. 5.
Identification of potential and actual caspase 3 cleavage sites in Rac1. (A) Caspase 3 treatment (5 ng/μl for the indicated times) and Western blot immunoassay (anti-Rac1 C terminus antibody) of purified, recombinant His-tagged Rac1 mutant proteins (100 ng/μl) containing Asp→Glu mutations at amino acid position 121, 11, or 47, or both 11 and 47. (B) Schematic diagram showing the key functional domains and caspase 3 cleavage sites of Rac1. Also shown are the truncated 20- and 16-kDa C-terminal proteins (G12 and G48) that result from caspase 3 cleavage at Asp11 and Asp47, respectively. (C) Schematic diagram of the tertiary structure of Rac1 (derived from the crystal structure of human Rac1-GMPPNP) (18) showing the critical Asp11 and Asp47 residues and the nucleotide and magnesium binding sites.