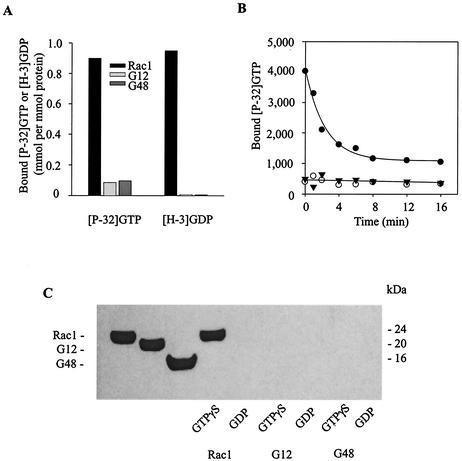
FIG. 7.
Inactivation of Rac1 by caspase 3 cleavage. (A) Recombinant purified His-tagged proteins representing wild-type Rac1 and the truncated proteins Rac1-G12 and Rac1-G48 were incubated with [γ-32P]GTP (left three bars) or [3H]GDP (right three bars) at room temperature for 30 min to achieve equilibrium. Bound nucleotide was quantified by a radioactive filter-binding assay as described under Materials and Methods. (B) Time courses of hydrolysis of [γ-P32]GTP by Rac1 (solid circles) and the truncated Rac1 G12 (open circles) and G48 (solid triangles) proteins. (C) Western blot immunoassay to test for binding of Rac1 and the G12 and G48 fragments to the p21-binding domain (PBD) of PAK1. Recombinant purified proteins (100 ng) (shown in the left three lanes) were loaded with GTP-γS or GDP and then incubated for 30 min at 4°C with 10 μg of GST-tagged PBD, followed by precipitation with glutathione-Sepharose beads. After each binding reaction, the proteins bound to the beads were eluted with SDS sample buffer, separated by SDS-PAGE, and visualized by anti-Rac1 (C-11) Western blot immunoassay.