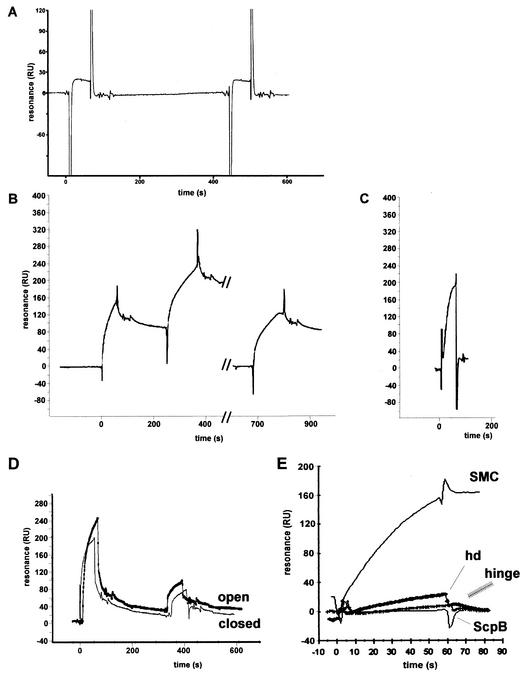
FIG. 6.
Surface plasmon resonance experiments. (A) ScpA (180 resonance units [RU]) was covalently immobilized on a Biacore chip. An equimolar mixture of the head domain and ScpB (2 μM each) was injected, followed by injection (at 450 s) of the head domain and ScpB (2 μM each) and a 500-bp linear DNA fragment. (B) A Streptavidin chip was coated with 350 RU of a 500-bp linear DNA fragment carrying biotin labels at both ends (closed). SMC (2 μM) was injected, followed by a second injection (2 μM, double amount at 250 s). The chip was washed with 50 mM NaOH, and SMC (2 μM, double amount at 680 s) was injected. (C) Same DNA as in panel B, except that the DNA was biotinylated only at the 3′ end (open). SMC (2 μM) was injected. Peaks flanking the binding curves were due to buffer fluctuations between the reference and the assay chamber at the beginning and end of each injection. (D) AbrB binding open or closed DNA. First injection, 12 μM AbrB; second injection, 6 μM AbrB. (E) Binding of different proteins to closed DNA: SMC (2 μM), head domain (hd; 2 μM), hinge (2 μM), or ScpB (20 μM).