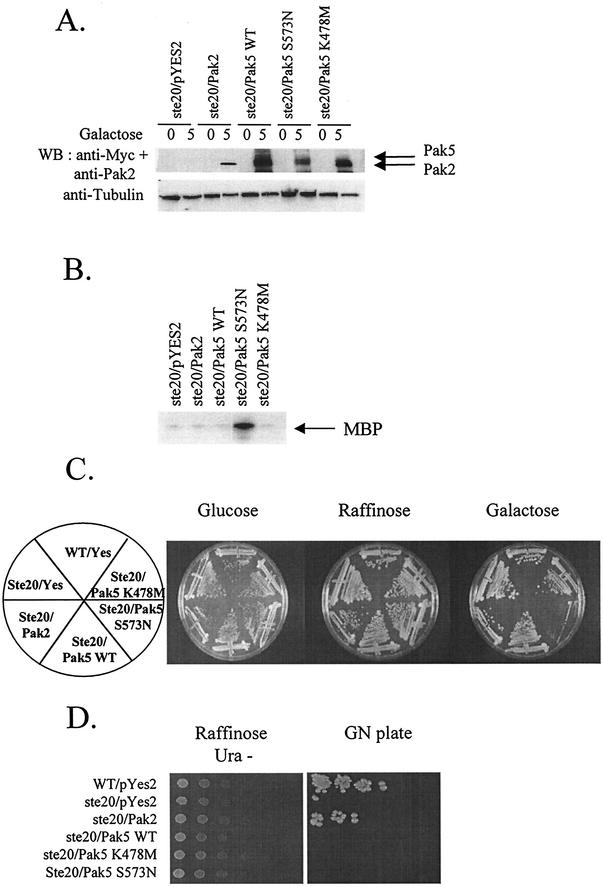
FIG. 1.
Complementation analysis of Pak5. ste20-null or a control strain of S. cerevisiae was transformed with a galactose-inducible expression vector bearing either no insert or a cDNA encoding Pak2, Myc-tagged WT Pak5, hyperactivated mutant (S573N), or K478 M (KD) mutant. Transformants were selected for growth in YNB URA− raffinose medium, galactose was added to 2% at time zero, and the cultures were induced for 5 h before harvesting. (A) Expression of the transgenes. Cells were lysed before and 5 h after induction, then assayed for protein expression by immunoblotting with monoclonal anti-Myc 9E10 or anti-Pak2. Equal protein levels was assessed using an antitubulin antibody. (B) Kinase assay. Cultures tested in panel A and obtained after 5 h of induction were lysed and immunoprecipitated with a polyclonal anti-Myc antibody or polyclonal anti-Pak2 and assayed for kinase activity as described in Materials and Methods. (C) Transformants were streaked onto YNB glucose, YNB raffinose, and YNB galactose URA− plates and incubated at 30°C before being photographed. (D) To perform semiquantitative mating assays, the transformants were serially diluted onto YNB raffinose URA− plates. After the colonies had grown sufficiently, the plates were replicated onto YNB Gal URA− for 5 h to allow expression of the transgenes. The cells were then replica-plated onto YEPD plates containing a lawn of the mating tester strain, and allowed to mate for six hours before being replicated onto GN plates. Mating efficiency was scored by the formation of prototrophic strains on the GN plates.