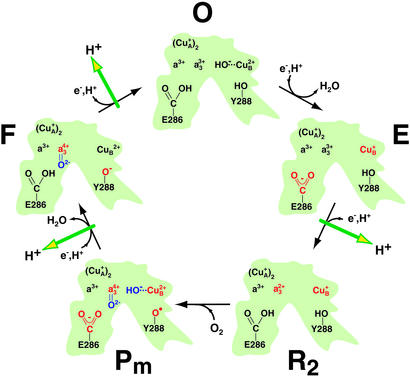
Fig. 2.
Qualitative overview of the molecular steps taking place in the active center of CcO during catalysis (adapted from refs. 9 and 37 and modified). In the initial state, all four redox centers (CuA, heme a, heme a3, and CuB) are oxidized (O). Successive electron input steps from cytochrome c initiate a series of molecular changes (in red) that finally lead to oxygen cleavage (in blue) and proton transfer across the membrane (green arrows). The first electron to the binuclear center reduces CuB to form the E state. The uptake of a second electron from cytochrome c reduces heme a3 (R2), and dioxygen can bind to the central iron of heme a3. Both initial electron transfer reactions are coupled to net proton uptake by the protein. In the Pm state, bound dioxygen is cleaved and tyrosine 288 (Y288) is proposed to be a radical. The uptake of the third electron with a proton forms the F state. The final step (F to O) is driven by the uptake of the fourth electron. It is concluded from the present work that E286 is protonated in the O, R2, and F state but deprotonated in the E and the Pm intermediate. The side chain of a tyrosine, presumed to be Y288, is a phenolate radical in Pm, a phenolate in F but a phenol in the other states. For simplicity, only those intermediates are included that are investigated in the present study. Although CcO maneuvers a total of eight protons during the cycle (four destined to form H2O and four pumped protons), stoichiometric balance of these protons is not shown in the figure because not all proton donors and acceptors within the protein have been identified.