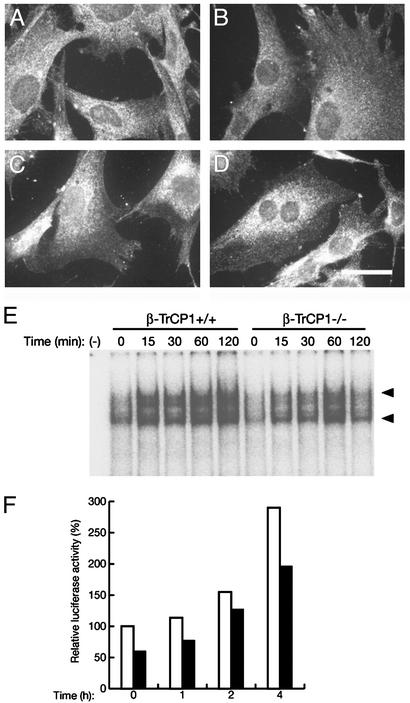
Fig. 3.
Reduced activity of NF-κB in β-TrCP1–/– cells. (A–D) Nuclear translocation of NF-κB in response to TNF-α. MEFs prepared from β-TrCP1+/+ (A and C) and β-TrCP1–/– (B and D) mice were incubated in the absence (A and B) or presence (C and D) of TNF-α. The cells then were subjected to immunofluorescence analysis with antibodies to the p65 (RelA) subunit of NF-κB. (Scale bar, 20 μm.) (E) DNA-binding activity of NF-κB. Thymocytes prepared from β-TrCP1+/+ and β-TrCP1–/– mice were stimulated for the indicated times with phorbol 12,13-dibutyrate and ionomycin, after which cell lysates were subjected to EMSA analysis with a probe specific for NF-κB. Arrowheads, positions of probe–protein complexes; –, incubation without cell lysate. (F) Activation of a luciferase reporter gene by NF-κB. MEFs derived from β-TrCP1+/+ (open bars) and β-TrCP1–/– (filled bars) mice were cotransfected with an NF-κB-dependent luciferase reporter construct and pRL-Tk. They then were stimulated with TNF-α for the indicated times, after which cell lysates were assayed for luciferase activities. Firefly luciferase activity was normalized by Renilla luciferase activity and then expressed as a percentage of the normalized value for β-TrCP1+/+ cells at time 0. Data are means of triplicates from an experiment that was repeated a total of three times with similar results.