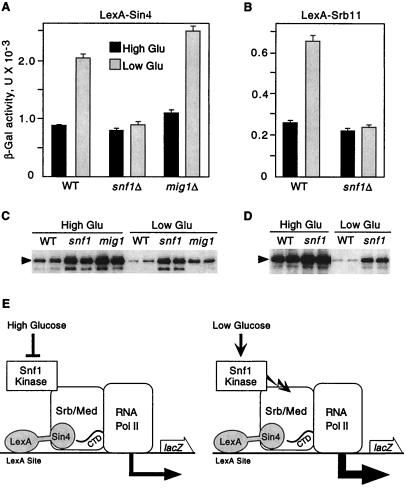
Figure 1.
Snf1 kinase stimulates transcription by artificially recruited holoenzyme. Strains were wild-type FY250 (WT), a snf1Δ10 derivative of FY250, and MCY3672 (mig1Δ), all with the S288C genetic background. Strains were transformed with the reporter pSH18-18 and a plasmid expressing LexA-Sin4 [pSK151; derivative of pEG202 (34)] (A and C) or LexA-Srb11 [pSK34; derivative of pLexA (1–202)+PL (35)] (B and D). (A and B) Transformants were grown to mid-log phase in 2% glucose (High Glu) and were shifted to 0.05% glucose (Low Glu) for 3 h. β-galactosidase activity was assayed, and values are averages for three transformants. (C and D) Crude extracts were prepared in duplicate from the assayed transformants, and immunoblot analysis with α-LexA was carried out. Each panel shows data from a single immunoblot. (E) Fusion of an Srb/mediator component to LexA recruits the holoenzyme to the reporter and causes high-level transcription. LexA-Sin4 is depicted here. The Snf1 kinase is shown in physical contact with the Srb/mediator complex (Srb/Med) based on data shown in Fig. 2. Activation of the Snf1 kinase by the low glucose signal further increases transcription a few fold. Jagged arrow represents Snf1 kinase activity. In this figure, the holoenzyme is depicted as the target of Snf1 kinase activity, but other components of the transcriptional apparatus are also possible targets.