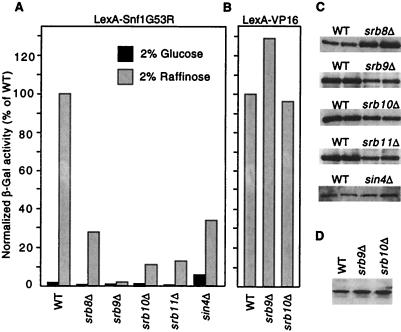
Figure 4.
Mutations in Srb/mediator genes reduce transcriptional activation by LexA-Snf1G53R. (A) Strains were cotransformed with the reporter pSH18-18 and pIT498 or pRJ216, which express LexA-Snf1G53R from the vectors pBTM116 (22) and pEG202 (34), respectively. Isogenic pairs were strain MCY3647 (wild-type, WT) and an srb8Δ derivative carrying pRJ216; FY250 (WT) and srb9Δ, srb10Δ, and srb11Δ disruptants carrying pIT498; and FY250 and a sin4Δ disruptant carrying pRJ216. β-galactosidase activity was assayed in cells grown to mid-log phase in 2% glucose or 2% raffinose plus 0.05% glucose. Values are averages for 3–10 transformants and are expressed as percent of the activity in the isogenic wild type grown in raffinose. Standard errors for raffinose-grown cultures were <20%. Elevated β-galactosidase activity was also observed in glucose-grown sin4Δ cells expressing LexA. (B) Strains used in A were transformed with a plasmid expressing LexA-VP16 (pLexA-VP16; a gift of S. Hollenberg, Oregon Health Sciences University, Portland, OR) and pSH18-18 or the related reporter plasmid 1840, which has only one LexA binding site (identical to 1145; ref. 40). β-galactosidase activity was assayed in cells grown in 2% raffinose plus 0.05% glucose as above. Results were similar for both reporters; data shown are averages of five transformants carrying 1840. (C) Crude extracts were prepared from two of the raffinose-grown WT and mutant transformants that were assayed in A. Proteins (15 μl) were separated by SDS/PAGE in 8% polyacrylamide and were immunoblotted with α-LexA to detect LexA-Snf1G53R. (D) Levels of LexA-VP16 protein in transformants were similarly assessed by immunoblotting.