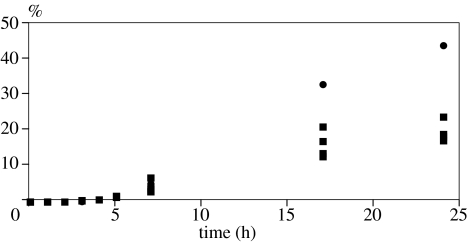
Figure 5.
Yield of uridine-5′-phosphite by reaction of uridine with ammonium phosphite (1 : 2 molar ratio) at 60°C. Solid squares, four sets of reactions giving the yields in the absence of urea; solid circles (upper series of data points), yields in the presence of urea (uridine : ammonium phosphate : urea=1 : 2 : 4). The initial time-lag observed for 5 h and less was probably due to the evaporation of water from the reaction. Under identical conditions as those shown here, ammonium phosphate produced no products (De Graaf & Schwartz 2005; reproduced with kind permission of Springer Science and Business Media).