Abstract
Studies of the responses of porcine pulmonary endothelial cells to acute hypertonic stress have been extended by examining the induction and underlying mechanisms of cell tolerance to both osmotic and heat stresses. Preliminary adaptation of these cells to 0.4osmol (kg H2O)−1 rendered them tolerant either to subsequent severe osmotic stress (0.7osmol (kg H2O)−1) or to subsequent severe heat shock (50 min at 49°C). In contrast, preliminary exposure of the cells to mild heat shock (44°C for 30 min) induced tolerance only to severe heat shock, not to hyperosmotic stress. Induction of tolerance to heat shock by either procedure correlated with the induced expression of heat shock protein 70 (HSP70). Induction of tolerance to hyperosmotic stress, on the other hand, was associated with the cellular accumulation of osmolytes, such as amino acids, betaine and myo-inositol, and did not correlate with the induced expression of HSP70. It also required a reduction in the final change of osmotic pressure applied to the cells, such that maximum cell shrinkage would not be much more than 40%. In general, therefore, HSP70 and compatible osmolytes have distinct roles in cellular adaptation to these stresses.
Many cells can survive temporary exposure to unusual, stressful, conditions, such as elevated osmolarity or temperature. If the stress is not too severe, some cells even adapt to such conditions and survive for long periods. For example, several kinds of mammalian cells can adapt to and survive in culture for days in hypertonic media of around 0.5osmol (kg H2O)−1, although they soon undergo apoptosis when exposed to osmolalities greater than about 0.6osmol (kg H2O)−1 (see Alfieri et al. 2002). Studies of cellular responses to heat shock, the stress probably most widely examined, have also revealed the phenomenon of acquired thermal tolerance. This is the ability of cells to tolerate a severe heat shock, too severe to be tolerated normally, after they have been ‘conditioned’ by preliminary exposure to a mild heat shock (Li & Werb, 1982; Kregel, 2002). Although it might not be the sole factor involved, the increased cellular expression and retention of heat shock proteins induced by the mild heat shock provides the obvious explanation for such acquired tolerance. This increased expression of some heat shock proteins occurs not only in cells subjected to thermal stress, but also in those experiencing a variety of other stresses, such as energy depletion or exposure to cytokines, endotoxins, heavy metals (reviewed by Kregel, 2002) or hypertonicity (Cohen et al. 1991). These observations raise the questions of whether cells can acquire tolerance to other stresses, besides heat shock, and of whether ‘cross-stress tolerance’ occurs: does preliminary exposure of cells to a mild form of one stress enable them to tolerate a severe form of a different stress?
Examples of both acquired osmotic tolerance and acquired cross-stress tolerance have been demonstrated with cultures of cells derived from kidney. For example, the former was shown when preliminary adaptation of Madin-Darby canine kidney (MDCK) cells to a medium adjusted to 0.6osmol (kg H2O)−1 with NaCl protected them against subsequent exposure to hyperosmotic solutions of urea (Neuhofer et al. 1998). The latter was demonstrated when cells derived from murine inner medullary collecting duct (mIMCD3) that had been ‘preconditioned with mild heat shock exhibited increased tolerance to hypertonic NaCl’, whilst their exposure to hypertonic stress similarly ‘conferred increased survival to thermal stress’ (Santos et al. 1998). Since the known responses of cells to these two different forms of stress are not identical, the extent of such cross-tolerance is likely to be limited. But where it does exist, comparison of the changes caused initially by each stress should enable the common features to be identified and their protective roles investigated. Induction of expression of heat shock proteins is the obvious response that seems to be common to most stresses, but complete adaptation of cultured cells to an abrupt increase of osmolarity clearly involves much more: usually, an ordered sequence of changes occur over a period of about 24 h (see below). Hence it is not clear that everything can be explained in terms of heat shock proteins and the questions arise of how common are such acquired tolerances and what molecular processes underlie them?
The adaptation of porcine pulmonary arterial endothelial cells to culture at 0.5osmol (kg H2O)−1 involves a series of sequential changes (Petronini et al. 2000; Alfieri et al. 2002). First, the immediate shrinkage of the cells in the hypertonic medium is followed by a slower regulatory volume increase, during which an increase in cell K+ concentration occurs. The cells then synthesize more membrane transporters for amino acids (ATA2; Alfieri et al. 2001) and, a few hours later, for betaine (BGT1), myo-inositol (SMIT) and taurine (TAUT) (Petronini et al. 2000; Alfieri et al. 2002). Thus, after initial shrinkage and recovery of volume through various ion fluxes, the cells first accumulate amino acids, and later the other osmolytes, as the cellular concentration of K+ gradually returns to normal. We have found, however, that with culture media of higher osmolality the cells fail to grow and many undergo apoptosis. Hence we have now investigated the possibility of acquired stress tolerance and found that preliminary adaptation of the cells to culture at 0.4osmol (kg H2O)−1 did indeed enable them to survive subsequent transfer to 0.7osmol (kg H2O)−1 conditions. Moreover, we found that cross-stress tolerance also occurs: adaptation to 0.4osmol (kg H2O)−1 rendered the cells tolerant to subsequent heat shock. On the other hand, preliminary exposure of the cells to mild heat shock provided protection only against subsequent severe heat shock, not against subsequent hyperosmotic challenge. Here we describe the limits of acquired tolerance and some details of the changes that occur during the successive exposure of the cells to these stresses.
Methods
Materials
[14C]Choline and myo-[3H]inositol were obtained from Amersham International (Amersham, UK). Choline oxidase, betaine, amino acids and myo-inositol were bought from Sigma Chemical Co. (Poole, UK). [14C]Betaine was prepared from [14C]choline by enzymic oxidation (Petronini et al. 1994). Media, fetal calf serum and antibiotics for culturing the cells were purchased from Gibco (Grand Island, NY, USA). Disposable plastics for laboratory use were obtained from Costar (Broadway, Cambridge, MA, USA). Other reagents of analytical grade were purchased from Sigma. Reagents for electrophoresis and blotting analysis were obtained from Bio-Rad Laboratories (Richmond, CA, USA). The antibody used in the Western blotting assay (monoclonal anti HSP72, clone C92F3 A-5; Stressgen, Victoria, BC, Canada) specifically recognized the inducible HSP70 isoform. Anti-actin monoclonal antibody was purchased from Sigma.
Cell culture
Pig pulmonary arteries were obtained from a local abattoir and the endothelial cells cultured as previously described (Petronini et al. 2000). The growth medium was minimal essential medium (MEM) containing 100Uml−1 penicillin, 100μgml−1 streptomycin and supplemented with 2mmol l−1 glutamine (final concentration 4mmoll−1), 2mmol l−1 sodium pyruvate, 10mmoll−1 Hepes (pH 7.4), 10% fetal calf serum, and 50μgml−1 endothelial cell growth factor. All cultures were kept in an incubator at 37°C in a water-saturated atmosphere of 5% CO2 in air. Cells used in this study were from early (up to the tenth) passages and were routinely checked for the expression of von Willebrand factor by immuno-cytochemistry with the use of rabbit antihuman von Willebrand Factor (DAKO Ltd, UK).
Experimental media
The media consisted of Earle's balanced salt solution (EBSSG) plus MEM amino acids, 100Uml−1 penicillin, 100μgml−1 streptomycin, 2mmoll−1 glutamine (final concentration 4mmoll−1), 2mmol l−1 sodium pyruvate, 10mmoll−1 Hepes (pH 7.4), MEM vitamins without myo-inositol, bovine serum albumin (0.1%, w/v), and 50μgml−1 endothelial cell growth factor, with betaine and myo-inositol (0.1mmoll−1) unless indicated otherwise. The amino acids were omitted for the experiments shown in Figs 7 and 11.
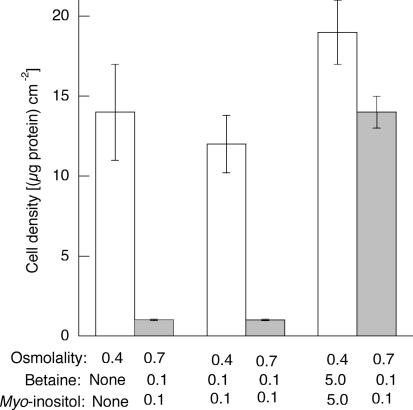
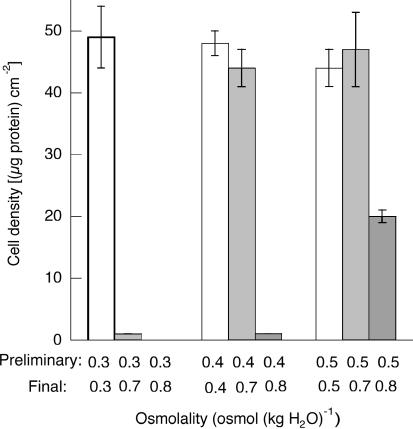
Figure 7. Effect of betaine and myo-inositol in the absence of amino acids.
Cells were incubated for 16 h in amino acid-free medium of 0.4osmol (kg H2O)−1, either without betaine and myo-inositol, or with each at the indicated concentrations (mmol (kg H2O)−1), and cell density was measured (0.4, open bars). The cells were then transferred to medium of 0.7osmol (kg H2O)−1 and incubated for a further 24 h before cell density was again measured (0.7 osmol (kg H2O)−1, grey bars). Osmolality was adjusted with sucrose. Mean values (±s.d.) from three measurements are given.
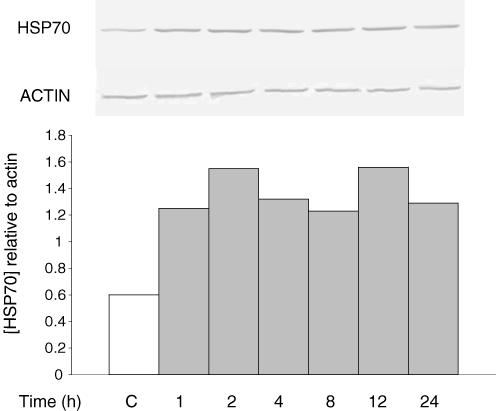
Figure 11. Lack of correlation with expression of HSP70.
Cells were incubated for 16 h in media of 0.3 or 0.4osmol (kg H2O)−1, with or without amino acids, and with betaine and myo-inositol each at 0.1 or 5mmol (kg H2O)−1, as indicated. The HSP70 content in samples was then measured by Western blotting and is expressed in the histogram relative to the actin content of the sample. Other samples were incubated for a further 24 h at 0.7osmol (kg H2O)−1, after which cell density was measured to test for survival.
The osmolalities of the media were checked with a vapour-pressure osmometer (Wescor). Normal growth medium was about 0.3osmol (kg H2O)−1. Hypertonic growth medium was made by the addition of sucrose to normal growth medium.
Determination of cell survival and growth
To test their viability under hypertonic conditions, endothelial cells were first seeded at a density of about 104 cells cm−2 and cultured for 2 days in the usual isotonic medium. Then the latter was replaced with experimental media. After experimental incubations cell density was assayed in terms of the protein content per dish (Alfieri et al. 2002).
Heat and osmotic stress
For mild heat stress (induction of thermal tolerance) the cells were incubated at 44°C for 30 min and then returned to 37°C for 6 or 16 h before being subjected to severe heat shock or hypertonicity. For mild osmotic stress (induction of osmotic tolerance) the cells were usually incubated at 0.4osmol (kg H2O)−1 for 16 h. For subsequent severe heat stress, the cells were incubated at 49°C for 50 min, then returned to 37°C for 24 h before their density was measured. For subsequent severe osmotic stress, the cells were incubated in media at 0.6–1osmol (kg H2O)−1 for 24 h before their density was measured.
Detection of apoptosis
Cell apoptosis was assessed both morphologically and in terms of increased caspase-3 activity. For the former, detached cells were stained with the dye Hoechst 33342 and their nuclear morphology examined by fluorescence microscopy. For determination of caspase activity, adherent endothelial cells were scraped from dishes and added to any cells that had detached spontaneously. The mixture of cells was washed by sedimentation and resuspension before being incubated in lysis buffer on ice for 10 min. Cell debris was then sedimented by centrifugation at 12 000 g for 2 min at 4°C and the caspase-3 activity of the supernatant solution was assayed colorimetrically with the use of a kit from MBL International Corporation (Watertown, MA, USA).
Ninhydrin-positive substances (NPS)
The intracellular content of NPS was measured by the method of Law & Turner (1987) and expressed as nmol (mg protein)−1.
Protein
Cell protein, precipitated by trichloroacetic acid, was dissolved in 0.5 m NaOH and its concentration determined by a dye-fixation method (Bio-Rad) using bovine serum albumin as standard (Bradford, 1976).
Measurement of solute uptake
The accumulation of betaine and myo-inositol by endothelial cells was monitored with the use of [14C]betaine or myo-[3H]inositol added to the incubation media. The incubations were stopped by removal of the medium and the cell monolayers quickly washed three times with Earle's balanced salt solution containing 0.1% glucose. Trichloroacetic acid (5%, w/v) was added to denature the cells and the radioactivity in samples of the acid extracts was measured by scintillation counting.
Western blotting
The procedures used for immunoblotting and protein analysis by 1-D PAGE were described in detail by Petronini et al. (1993).
Results
Limitation of adaptation of endothelial cells to hypertonicity
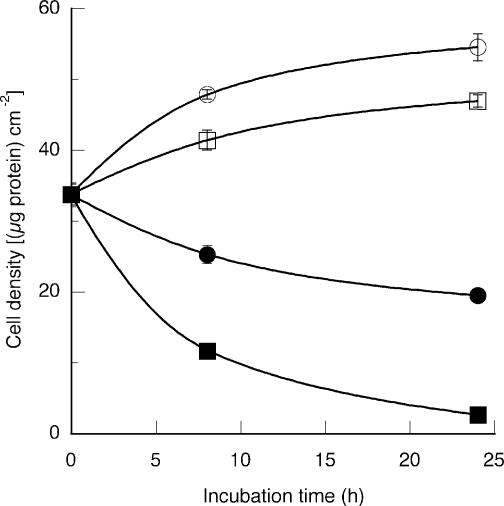
The morphology and protein content of porcine pulmonary endothelial cells were monitored during the incubation in media of various osmolalities. Figure 1 illustrates the effects of hypertonicity on cell growth and survival of their transfer from isotonic (0.3osmol (kg H2O)−1) medium to hypertonic media in the range 0.5–0.7osmol (kg H2O)−1. Although the cells adapted to the 0.5osmol (kg H2O)−1 medium, they failed to grow at 0.6 or 0.7osmol (kg H2O)−1, many rounding up and detaching from the plates. Staining with the DNA dye Hoechst 33342 revealed that most of the detached cells had clear apoptotic morphology: their chromatin either had the classic semilunar appearance or had collapsed into patches and dense spheres. In keeping with these observations, the caspase-3 activity of the cells increased markedly during their incubation at 0.6 and 0.7 osmol (kg H2O)−1, but was barely detectable under 0.3 or 0.5osmol (kg H2O)−1 conditions (data not shown).
Figure 1. Effects of osmolality on cell growth.
Cells were seeded and cultured for 48 h in isotonic (0.3osmol (kg H2O)−1) medium before being transferred to a hypertonic (0.5, 0.6 or 0.7osmol (kg H2O)−1) medium. Osmolality was adjusted with sucrose. Culture was then continued for the indicated times and cell growth estimated by measurement of the amount of cell protein. Mean values (±s.d.) from three measurements are given. ○, 0.3; □, 0.5; •, 0.6; ▪, 0.7osmol (kg H2O)−1.
Induction of tolerance to hypertonicity
In contrast to the results shown in Fig. 1, the endothelial cells became tolerant to incubation at 0.7osmol (kg H2O)−1 after they had first been exposed to media of 0.4–0.5osmol (kg H2O)−1 for 24 h (Fig. 2). This was not just a temporary protection because the cells remained viable and grew, albeit slowly, in this extremely hypertonic medium for at least 5 days after a preliminary exposure to 0.4osmol (kg H2O)−1 for 16 h. In fact, the time required at 0.4osmol (kg H2O)−1 to render the cells maximally tolerant to subsequent incubation at 0.7osmol (kg H2O)−1 was only about 8–9 h (Fig. 3). However, the induced tolerance was limited because cells adapted to 0.4osmol (kg H2O)−1 failed to tolerate subsequent incubation at osmolalities higher than 0.7osmol (kg H2O)−1 (data not shown).
Figure 2. Induction of osmotic tolerance.
Cells were incubated for 24 h in medium of the indicated osmolality and cell density was measured in terms of the amount of protein per unit area (○). The cells were then transferred to medium of 0.7osmol (kg H2O)−1 and incubated for a further 24 h before cell density was again measured (•). Osmolality was adjusted with sucrose. Mean values (±s.d.) from three measurements are given.
Figure 3. Time required for adaptation.
Cells were incubated for the indicated time in medium of 0.4osmol (kg H2O)−1 and cell density was measured in terms of the amount of protein per unit area (○). The cells were then transferred to medium of 0.7osmol (kg H2O)−1 and incubated for a further 24 h before cell density was again measured (•). Osmolality was adjusted with sucrose. Mean values (±s.d.) from three measurements are given.
Induction of tolerance to heat
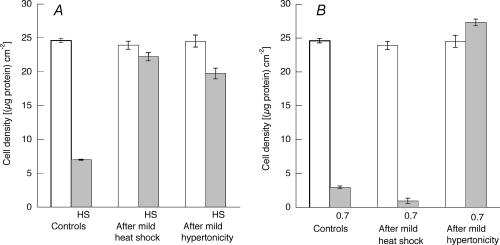
It is well established that exposure of many kinds of cells to mild heat shock elicits a transient state of ‘acquired thermo-tolerance’ and, as shown in Fig. 4A, preliminary incubation of these endothelial cells at 44°C for 30 min protected them against more severe heat shock (50 min at 49°C). In addition, however, these cells show some cross-stress tolerance, because their preliminary adaptation to 0.4osmol (kg H2O)−1 similarly protected them against severe heat shock (Fig. 4A). In contrast, conditioning the cells with mild heat shock did not protect them against severe hypertonic shock (Fig. 4B). Nor did conditioning with mild heat shock affect the survival (about 45%) of cells subsequently exposed to the lesser stress of 0.6osmol (kg H2O)−1 (data not shown).
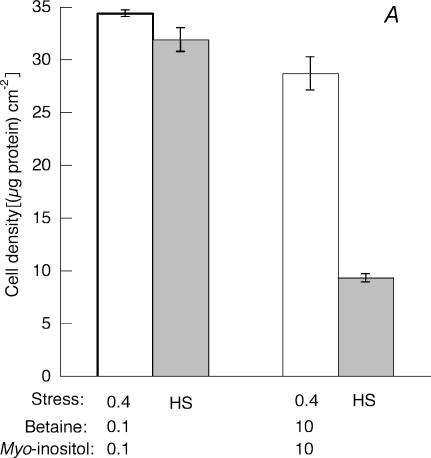
Figure 4. Partial induction of cross-stress tolerance.
Control cells were maintained at 37°C and 0.3osmol (kg H2O)−1 while experimental cells were first exposed either to mild heat shock (44°C for 30 min followed by 37°C for 6 h) or to mild hypertonicity (16 h at 0.4osmol (kg H2O)−1). Cell density was measured in terms of protein content (open bars). Samples were then subjected to severe heat shock (49°C for 50 min) and incubated for 24 h at 37°C before cell density was again measured (A, HS, grey bars). Or samples were then incubated at 0.7osmol (kg H2O)−1 for 24 h before cell density was again measured (B, 0.7, shaded bars). Mean values (±s.d.) from three measurements are given.
Loss of tolerance to hypertonicity
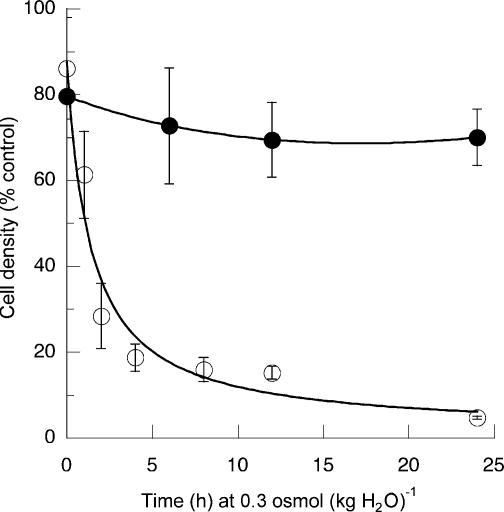
The induced tolerance of the cells to culture at 0.7osmol (kg H2O)−1 was lost over a period of a few hours when they were transferred back from 0.4osmol (kg H2O)−1 medium to isotonic (0.3osmol (kg H2O)−1) medium (Fig. 5). On the other hand, Fig. 5 also shows that the tolerance to heat shock induced by culture at 0.4osmol (kg H2O)−1 was not readily lost when the cells were returned to isotonic medium.
Figure 5. Loss of tolerance to hypertonicity but not to heat.
Cells were incubated for 16 h in medium of 0.4osmol (kg H2O)−1 and then transferred to medium of 0.3osmol (kg H2O)−1 for the indicated time. Osmolality was adjusted with sucrose. Cell density was then measured in terms of the amount of protein per unit area. Samples were then subjected either to severe heat shock (49°C for 50 min followed by 24 h at 37°C) or to hypertonicity (0.7 osmol (kg H2O)−1 for 24 h before cell density was again measured. The latter values are expressed as a percentage of the corresponding values before the final stress. ○, final exposure to hypertonicity, mean values (±s.d.) from six measurements. •, final exposure to heat shock; mean values (±s.d.) from three measurements.
Underlying processes
The sequence of changes that occur when the cells adapt to from culture at 0.3osmol (kg H2O)−1 to culture at 0.5osmol (kg H2O)−1, as outlined in the introduction, is mimicked when the cells are transferred from 0.3 to 0.4osmol (kg H2O)−1, or from the latter to 0.7osmol (kg H2O)−1 (results not shown). Hence there are two main factors that might underlie the induction of tolerance to hypertonic culture: (1) a limitation on the extent of shrinkage that the cells can tolerate, and (2) the cells' acquisition of compatible osmolytes.
Cell shrinkage
If the cells behave as perfect osmometers, their initial shrinkage on transfer from 0.4 to 0.7osmol (kg H2O)−1 would be 43%, not much more than the 40% expected after transfer from 0.3 to 0.5osmol (kg H2O)−1. If this is about the maximum shrinkage the cells can tolerate, their failure to adapt from 0.3 to much more than 0.5osmol (kg H2O)−1 (Fig. 1) or from 0.4 to more than 0.7osmol (kg H2O)−1 is understandable. This interpretation suggests that cells adapted to culture at 0.5osmol (kg H2O)−1 should tolerate transfer to 0.8osmol (kg H2O)−1, because this should involve an initial shrinkage of no more than 38%. Cells cultured at 0.5osmol (kg H2O)−1 for 16 h, however, failed to survive subsequent incubation at 0.8osmol (kg H2O)−1. Hence, although there must be a limit to the extent of shrinkage the cells can tolerate, this is not the only factor involved.
Importance of osmolytes
The time course for the acquisition of osmotic tolerance (Fig. 3) is consistent with the notion that the accumulation of some compatible osmolytes was necessary. In view of the known sequences of changes that occur when the cells are transferred to hypertonic medium, during their first 8 h at 0.4osmol (kg H2O)−1 they would have accumulated mainly amino acids, with smaller amounts of betaine and myo-inositol. Hence, we checked to see if the loss of the acquired tolerance when the cells were transferred back from 0.4 to 0.3osmol (kg H2O)−1 (Fig. 5) was associated with the loss of these osmolytes. The results in Fig. 6 show that the cell content of NPS (mainly amino acids) did indeed decrease in parallel with the loss of acquired osmotic tolerance. On the other hand, Fig. 6 also shows that neither betaine nor myo-inositol left the cells quickly under these conditions: no loss at all occurred during the 24 h monitored. (Note that, for these measurements, betaine and myo-inositol were each present at a concentration of 0.1mmoll−1 in the 0.4osmol (kg H2O)−1 medium.) The lack of importance of betaine and myo-inositol in this situation was confirmed by showing that their absence from the 0.4osmol (kg H2O)−1 conditioning medium had no effect on the cells' ability to tolerate subsequent incubation at 0.7osmol (kg H2O)−1 (data not shown). On the other hand, when amino acids were removed from the 0.4osmol (kg H2O)−1 medium, the presence of betaine and myo-inositol was important. The cells, of course, did not grow well in the absence of amino acids, but a reasonable proportion did survive incubation for 16 h. These survivors did not acquire osmotic tolerance, however, unless betaine and myo-inositol had been added to give a high final concentration (each 5mmoll−1) in the conditioning medium in place of the amino acids (Fig. 7).
Figure 6. Partial loss of osmolytes.
Cells were incubated for 16 h in medium of 0.4osmol (kg H2O)−1 and then transferred to medium of 0.3osmol (kg H2O)−1 for the indicated time. The cellular content of ninhydrin-specific solutes was then measured (□). In replicate incubations, radioactively labelled betaine (▪) and myo-inositol (•) were used to monitor their cellular concentrations. Mean values (±s.d.) from three measurements are given.
In view of the last result, we checked the effect of the addition of betaine and myo-inositol (each at a final concentration of 5mmoll−1) to the normal media, containing amino acids, used for the preliminary conditioning incubations. As shown in Fig. 8, cells incubated under isotonic conditions (0.3osmol (kg H2O)−1) with these high concentrations of betaine and myo-inositol were still unable to tolerate subsequent incubation at 0.7osmol (kg H2O)−1. Similarly, preliminary incubations with the high concentrations of osmolytes at 0.4osmol (kg H2O)−1 still provided no tolerance to subsequent incubations at 0.8osmol (kg H2O)−1. But preliminary incubation at 0.5osmol (kg H2O)−1 with the extra betaine and myo-inositol did provide partial tolerance to subsequent incubation at 0.8 osmol (kg H2O)−1.
Figure 8. Extension of tolerance.
Cells were first incubated for 16 h in media of 0.3, 0.4 or 0.5osmol (kg H2O)−1 containing 5mmol (kg H2O)−1 each of betaine and myo-inositol. They were then transferred to media of 0.7 or 0.8osmol (kg H2O)−1, containing the normal concentrations of betaine and myo-inositol (each at 0.1mmol (kg H2O)−1) and incubated for a further 24 h. Cell densities were measured after each incubation. Osmolality was adjusted with sucrose and mean values (±s.d.) from three measurements are given.
Cross-stress tolerance
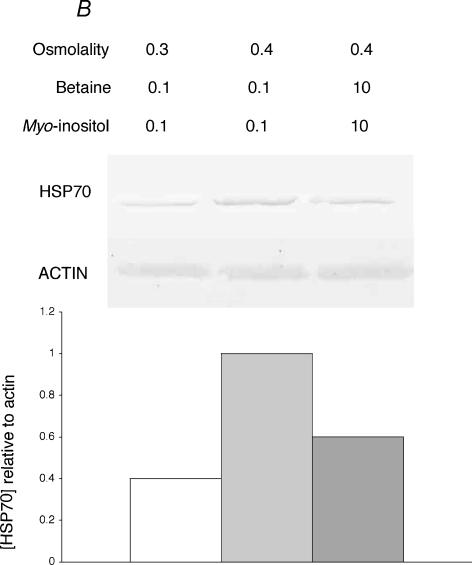
The hypertonic induction of tolerance to heat shock correlated with both the accumulation of compatible osmolytes and the increased production of HSP70. For example, Fig. 6 shows that the cellular content of betaine and myo-inositol, which had increased during preliminary incubation of the cells at 0.4osmol (kg H2O)−1, remained constant through the subsequent incubation at 0.3osmol (kg H2O)−1. Similarly, when the cellular content of inducible HSP70 was monitored during an experiment similar to those described in Figs 5 and 6, the results shown in Fig. 9 were obtained. The HSP70 content clearly increased during the preliminary incubation of the cells at 0.4osmol (kg H2O)−1 and then remained high through the subsequent incubation at 0.3osmol (kg H2O)−1, thus paralleling the induced tolerance to heat shock shown in Fig. 5. This link between the accumulation of compatible osmolytes and of HSP70 can, however, be broken by the addition of a high concentration of osmolyte to the conditioning hypertonic medium. Under these conditions, the cells accumulate the osmolyte and this somehow suppresses the induction of HSP70 expression. When betaine and myo-inositol were each present at 10mmoll−1 during exposure of the cells to 0.4osmol (kg H2O)−1, induction of HSP70 expression and induction of tolerance to heat shock were both reduced (Fig. 10). Under this condition therefore when compatible osmolytes were abundant, failure to induce tolerance to heat shock still paralleled failure to induce expression of HSP70.
Figure 9. Induction and retention of HSP70.
Cells were incubated for 16 h in medium of 0.4osmol (kg H2O)−1 and then transferred to medium of 0.3osmol (kg H2O)−1 for the indicated time, as in Figs 5 and 6. Control cells (C, open bar) were incubated in isotonic (0.3osmol (kg H2O)−1) medium. The cellular content of HSP70 was then monitored by Western blotting (upper panel) and is expressed in the histogram relative to the actin content of the sample (lower panel).
Figure 10. Decreased tolerance to heat shock.
A, cells were incubated for 16 h in medium of 0.4osmol (kg H2O)−1 with betaine and myo-inositol each either at the normal 0.1 mmol (kg H2O)−1), or increased to 10 mmol (kg H2O)−1, and cell density was measured (0.4, open bars). Samples were then subjected to severe heat shock (49°C for 50 min) and incubated for 24 h at 37°C before cell density was again measured (HS, grey bars). Osmolality was adjusted with sucrose. Mean values (±s.d.) from three measurements are given. B, the HSP70 content of the cells at the end of the 16 h incubation at 0.4 osmol (kg H2O)−1 was detected by Western blotting and compared with that of cells maintained in isotonic medium. It is expressed in the histogram relative to the actin content of the sample.
In contrast to the situation just described, there was no overall correlation between cellular content of HSP70 and tolerance to hypertonicity. In addition to the results given in Figs 5 and 9, data from a number of other conditions are given in Fig. 11. Lanes 1 and 2 show induction of HSP70 expression when cells acquired osmotic tolerance during preliminary incubation at 0.4osmol (kg H2O)−1. Lane 3, however, shows a similar induction of HSP70 expression without induction of osmotic tolerance, when amino acids had been removed from the conditioning medium. Again, the opposite is shown by lanes 4 and 5: induction of osmotic tolerance in the absence of increased expression of HSP70, when amino acids in the conditioning medium had been replaced with high concentrations of betaine and myo-inositol.
Discussion
Acquired osmotic tolerance
As noted above, preliminary incubation of cells at 0.4 instead of 0.3osmol (kg H2O)−1 necessarily reduces the stress of subsequent transfer to 0.7osmol (kg H2O)−1, because the imposed cell shrinkage and its consequences are significantly less. The possible candidates for the mediation of the responses to cell shrinkage include increase in cellular ionic strength, increase in ‘molecular crowding’ (Garner & Burg, 1994), and deformation of the plasma membrane or cytoskeleton. Although the relative importance of these possibilities remains unclear, the smaller the cell shrinkage, the smaller each of these will be. Thus the cells' acquisition of osmotic tolerance in this way is fundamentally different from their acquisition of thermal tolerance. On the other hand, the results described above show that the extent of cell shrinkage is not the sole factor involved: the cells must also accumulate sufficient compatible osmolytes in order to tolerate subsequent more severe hypertonicity. This is evident from Fig. 8, which illustrates both points. First, the various transfers of cells from 0.3 to 0.7osmol (kg H2O)−1, and from 0.4 to 0.7 and 0.8osmol (kg H2O)−1, showed that they did not tolerate hypertonic stress that, theoretically, would cause much more than about 40% shrinkage. Second, although transfer from 0.5 to 0.8osmol (kg H2O)−1 did not cause too much shrinkage, the cells required sufficient compatible osmolytes during preliminary incubation at 0.5osmol (kg H2O)−1 for any of them to tolerate the subsequent transfer to 0.8osmol (kg H2O)−1.
Thus the necessary conditions for adaptation appear to be simply that (a) the increments in osmolarity must be small enough, and (b) the cells must be able to accumulate sufficient compatible osmolytes. This conclusion suggests that stepwise, or gradual, adaptation from isotonic conditions might also enable the cells to survive even more severe hypertonic stress. The latter prediction certainly seems to be true for some kidney-derived cells, because Cai et al. (2002) showed that almost 90% of mouse inner medullary epithelial cells (mIMCD) remained viable when their culture medium was slowly changed, over a period of 20 h, from 0.6 to 1.6osmol (kg H2O)−1, compared with only 30% survival when the change was abrupt.
The example of acquired osmotic tolerance in which preliminary adaptation of MDCK cells to a medium adjusted to 0.6osmol (kg H2O)−1 with NaCl protected them against subsequent exposure to hyperosmotic solutions of urea (Neuhofer et al. 1998) seems to have a different basis. Further studies by Neuhofer et al. (1999) revealed a correlation between the cells' acquisition of osmotic tolerance and their expression of heat shock proteins during the preliminary conditioning treatment. We, in contrast, found no general correlation between the expression of HSP70 and adaptation to hypertonicity. For example, Figs 5 and 9 show that, although tolerance to hypertonicity paralleled induction of HSP70 expression during exposure of the cells to 0.4osmol (kg H2O)−1, loss of tolerance to hypertonicity during their subsequent incubation at 0.3osmol (kg H2O)−1 was not accompanied by loss of expression of HSP70. The similar discrepancies shown in Fig. 11 have been described above. Possible explanations of these different findings are the difference between the kinds of cells used and the difference in the final osmotic stress. The use of urea with the cells derived from kidney is relevant physiologically; but, in contrast to sucrose, it readily enters cells and can have adverse effects on protein conformation. Heat shock proteins might have a particularly important protective role under these conditions.
Acquired thermal tolerance
Either preliminary mild heat shock or preliminary exposure to hypertonic medium made these endothelial cells tolerant to subsequent severe heat shock. Each of these conditioning treatments induce the expression of heat shock proteins, but, unlike hypertonicity, there is no evidence that heat shock can induce the expression of the transporters for amino acids or compatible osmolytes such as betaine. It does not induce mRNA for ATA2 in mIMCD3 cells (Nahm et al. 2002) or for BGT1 in MDCK cells (Sheikh-Hamad et al. 1994). Moreover, although heat shock (44°C for 30 min) did cause an immediate increase (about 70%) of NPS in these endothelial cells, this disappeared during the first 4 h of recovery at 37°C (not shown). Thus the factor common to both stresses was the induction of heat shock proteins, and it appears that these were sufficient to make the cells tolerant to heat shock. This interpretation is supported by the fact that when the hypertonic medium was supplemented with high concentrations of betaine and myo-inositol, induction of thermal tolerance was largely suppressed (Fig. 10), so there is no evidence that compatible osmolytes alone afford protection against heat shock. Hence, although it remains possible that compatible osmolytes might support the action of heat shock proteins, both the normal response of cells to heat shock and their acquisition of thermal tolerance appear to be relatively straightforward processes that depend on the expression of heat shock proteins. Since many other stresses similarly induce the expression of heat shock proteins (Kregel, 2002), it seems likely that cells may acquire thermal tolerance during exposure to a number of different stresses
Cross-stress tolerance
The results just discussed clearly provided an example of cross-stress tolerance, when cells adapted to culture at 0.4osmol (kg H2O)−1 became tolerant to subsequent heat shock. Santos et al. (2003) similarly showed recently that mIMCD3 cells rendered osmotically tolerant by gradual adaptation to hypertonic media were also more resistant to a variety of other stressful conditions. But we could find no evidence for the other possibility, that preliminary heat shock could render the cells tolerant to subsequent hypertonicity. Preliminary heat shock appeared to induce only the expression of heat shock proteins and, as pointed out above, there was no overall correlation between HSP expression and tolerance to hypertonicity. On the other hand, the fact that hypertonicity induces the expression of HSP indicates that it has a role in protection against this stress. Perhaps HSP prevents apoptosis (Garrido et al. 2001) by affording temporary protection to other proteins from the increase in ionic strength and molecular crowding caused by cell shrinkage, but subsequent increased uptake of osmolytes is still necessary for survival of the cells in a hypertonic medium.
It is difficult to explain the apparent discrepancy between these findings and those of Santos et al. (1998), who found that preliminary heat shock increased the survival of mIMCD3 cells (from about 28 to 58%) during their subsequent exposure to hypertonicity. One minor difference between the studies was the duration of the final osmotic stress, following preliminary mild heat shock: Santos et al. (1998) used 8 h, whereas we used 24 h. Apart from such quantitative differences, a cell-specific effect is the most likely explanation.
Physiological significance
As we have discussed previously (Alfieri et al. 2002), the physiological significance of these endothelial cells, and several other types of cell, of being able to adapt so well to hypertonicity remains speculative. It may be important mainly under certain pathological conditions that affect cell volume (McManus et al. 1995). The cells certainly appear to have invested too much in these responses to hypertonicity for them to be either physiologically irrelevant or ignored.
Acknowledgments
We thank the Ministero della Istruzione, della Università e della Ricerca Scientifica, Rome, Italy for financial support.
References
- Alfieri RR, Cavazzoni A, Petronini PG, Bonelli MA, Caccamo AE, Borghetti AF, Wheeler KP. Compatible osmolytes modulate the responses of porcine endothelial cells to hypertonicity and protect them from apoptosis. J Physiol. 2002;540:499–508. doi: 10.1113/jphysiol.2001.013395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri RR, Petronini PG, Bonelli MA, Caccamo AE, Cavazzoni A, Borghetti AF, Wheeler KP. Osmotic regulation of ATA2 mRNA expression and amino acid transport System A activity. Biochem Biophys Res Com. 2001;283:174–178. doi: 10.1006/bbrc.2001.4729. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cai Q, Michea L, Andrews P, Zhang Z, Rocha G, Dmitrieva N, Burg MB. Rate of increase of osmolarity determines osmotic tolerance of mouse inner medullary epithelial cells. Am J Physiol. 2002;283:F792–F798. doi: 10.1152/ajprenal.00046.2002. [DOI] [PubMed] [Google Scholar]
- Cohen DM, Wasserman JC, Gullans SR. Immediate early gene and HSP70 expression in hyperosmotic stress in MDCK cells. Am J Physiol. 1991;261:C594–C601. doi: 10.1152/ajpcell.1991.261.4.C594. [DOI] [PubMed] [Google Scholar]
- Garner MM, Burg MB. Macromolecular crowding and confinement in cells exposed to hypertonicity. Am J Physiol. 1994;266:C877–C892. doi: 10.1152/ajpcell.1994.266.4.C877. [DOI] [PubMed] [Google Scholar]
- Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J App Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Law RO, Turner DPJ. Are ninhydrin-positive substances volume-regulatory osmolytes in rat renal papillary cells? J Physiol. 1987;386:45–61. doi: 10.1113/jphysiol.1987.sp016521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med. 1995;333:1260–1267. doi: 10.1056/NEJM199511093331906. [DOI] [PubMed] [Google Scholar]
- Nahm O, Woo SK, Handler JS, Kwon HM. Involvement of multiple kinase pathways in stimulation of gene transcription by hypertonicity. Am J Physiol. 2002;282:C49–C58. doi: 10.1152/ajpcell.00267.2001. [DOI] [PubMed] [Google Scholar]
- Neuhofer WM, Müller E, Burger-Kentischer A, Frack ML, Thurau K, Beck FX. Pretreatment with hypertonic NaCl protects MDCK cells against high urea concentrations. Pflügers Arch. 1998;435:407–414. doi: 10.1007/s004240050531. [DOI] [PubMed] [Google Scholar]
- Neuhofer WM, Müller E, Burger-Kentischer A, Frack ML, Thurau K, Beck FX. Inhibition of NaCl-induced heat shock protein 72 expression renders MDCK cells susceptible to high urea concentrations. Pflügers Arch. 1999;437:611–616. doi: 10.1007/s004240050824. [DOI] [PubMed] [Google Scholar]
- Petronini PG, Alfieri RR, De Angelis E, Campanini C, Borghetti AF, Wheeler KP. Different HSP70 expression and cell survival during adaptive responses of 3T3 and transformed 3T3 cells to osmotic stress. Br J Cancer. 1993;67:493–499. doi: 10.1038/bjc.1993.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronini PG, Alfieri RR, Losio MN, Caccamo AE, Cavazzoni A, Bonelli MA, Borghetti AF, Wheeler KP. Induction of BGT1 and amino acid System A transport activities in endothelial cells exposed to hyperosmolarity. Am J Physiol. 2000;279:R1580–R1589. doi: 10.1152/ajpregu.2000.279.5.R1580. [DOI] [PubMed] [Google Scholar]
- Petronini PG, De Angelis E, Borghetti AF, Wheeler KP. Osmotically inducible uptake of betaine via amino acid transport system A in SV-3T3 cells. Biochem J. 1994;300:45–50. doi: 10.1042/bj3000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos BC, Chevaile A, Kojima K, Gullans SR. Characterisation of the Hsp110/SSE gene family response to hyperosmolality and other stresses. Am J Physiol. 1998;274:F1054–F1061. doi: 10.1152/ajprenal.1998.274.6.F1054. [DOI] [PubMed] [Google Scholar]
- Santos BC, Pullman JM, Chevaile A, Welch WJ, Gullans SR. Chronic hyperosmolarity mediates constitutive expression of molecular chaperones and resistance to injury. Am J Physiol. 2003;284:F564–F574. doi: 10.1152/ajprenal.00058.2002. [DOI] [PubMed] [Google Scholar]
- Sheikh-Hamad D, Garcia-Perez A, Ferraris JD, Peters EM, Burg MB. Induction of gene expression by heat shock versus osmotic stress. Am J Physiol. 1994;267:F28–F34. doi: 10.1152/ajprenal.1994.267.1.F28. [DOI] [PubMed] [Google Scholar]