Abstract
Store-mediated Ca2+ entry (SMCE) is a major mechanism for Ca2+ influx in non-excitable cells. Recently, a conformational coupling mechanism allowing coupling between transient receptor potential channels (TRPCs) and IP3 receptors has been proposed to activate SMCE. Here we have investigated the role of two soluble N-ethylmaleimide-sensitive-factor attachment protein receptors (SNAREs), which are involved in membrane trafficking and docking, in SMCE in human platelets. We found that the synaptosome-associated protein (SNAP-25) and the vesicle-associated membrane proteins (VAMP) coimmunoprecipitate with hTRPC1 in platelets. Treatment with botulinum toxin (BoNT) E or with tetanus toxin (TeTx), induced cleavage and inactivation of SNAP-25 and VAMPs, respectively. BoNTs significantly reduced thapsigargin- (TG) and agonist-evoked SMCE. Treatment with BoNTs once SMCE had been activated decreased Ca2+ entry, indicating that SNAP-25 is required for the activation and maintenance of SMCE. In contrast, treatment with TeTx had no effect on either the activation or the maintenance of SMCE in platelets. Finally, treatment with BoNT E impaired the coupling between naturally expressed hTRPC1 and IP3 receptor type II in platelets. From these findings we suggest SNAP-25 has a role in SMCE in human platelets.
Stimulation of non-excitable cells with agonists results in a rise in the intracellular free calcium concentration ([Ca2+]i) due to Ca2+ release from intracellular stores and Ca2+ entry across the plasma membrane (PM) (Rink & Sage, 1990). A major mechanism for Ca2+ entry in non-excitable cells is store-mediated Ca2+ entry (SMCE), a process controlled by the filling state of the intracellular Ca2+ stores (Putney, 1990); however, the mechanism by which the content of the intracellular stores is communicated to the plasma membrane is still unclear. Several hypotheses fall into two main categories: indirect coupling, which assumes the generation of a small messenger molecule that directly or through different biochemical events opens channels or inserts channels into the plasma membrane so facilitating Ca2+ entry, and direct coupling, which suggests a physical interaction between a Ca2+-permeable channel in the plasma membrane and a protein in the endoplasmic reticulum (ER) (Parekh & Penner, 1997). In the last few years, studies by us and others have provided compelling evidence favouring a conformational coupling-based model as the mechanism activating SMCE in several non-excitable cells, including smooth muscle, platelets and pancreatic acinar cells (Patterson et al. 1999; Rosado et al. 2000b; Redondo et al. 2003). This conformational coupling model proposes that Ca2+ store depletion leads to trafficking of portions of the ER towards the PM to facilitate coupling between proteins in the two membranes. Since the discovery of mammalian homologues of the Drosophila transient receptor potential channel (TRPC), these proteins have been presented as candidates for the conduction of SMCE (Zhu et al. 1996; Putney & McKay, 1999; Liu et al. 2000) and several studies have reported functional coupling between various IP3 receptor (IP3R) isoforms and TRPCs in transfected cells (Boulay et al. 1999; Putney, 1999; Lockwich et al. 2000). We have demonstrated a physical and reversible coupling between the type II IP3R and naturally expressed hTRPC1 in human platelets, which is rapidly activated de novo by depletion of the intracellular Ca2+ stores (Rosado & Sage, 2000b; Brownlow & Sage, 2003). In this model, as with secretion, reorganization of the actin cytoskeleton, particularly those filaments located beneath the PM, plays a key regulatory role in the activation and maintenance of SMCE (Rosado & Sage, 2001a).
Studies conducted to investigate the nature of the secretory process revealed that a number of proteins, collectively termed soluble N-ethylmaleimide-sensitive-factor attachment protein receptors (SNAREs), act as membrane recognition molecules and acceptors for vesicle trafficking, docking and fusion (Duman & Forte, 2003). Among these proteins, the synaptosome-associated protein (SNAP-25) and the vesicle-associated membrane proteins (VAMPs) are components of a multiprotein complex proposed to mediate vesicle docking (Söllner et al. 1993). These proteins mediate the formation of extremely stable complexes between adjacent membranes, which are even SDS resistant, bringing the membranes into close apposition (Duman & Forte, 2003). Since the conformational coupling model for the activation of SMCE is based on the physical interaction between the membrane of the ER and the PM, the aim of the present study is to investigate the possible involvement of SNAP-25 and VAMPs in Ca2+ entry in human platelets, where a conformational coupling has been proposed to account for the activation of SMCE.
Methods
Materials
Fura-2 acetoxymethyl ester (fura-2 AM) and calcein were from Molecular Probes (Leiden, the Netherlands). Apyrase (grade VII), aspirin, bovine serum albumin, thrombin, tetanus toxin, botulinum toxin E, phenylmethylsulphonyl fluoride, leupeptin, benzamidine, paraformaldehyde, thapsigargin (TG), Ponceau stain and ionomycin (Iono) were from Sigma (Madrid, Spain). Anti-SNAP-25 antibody (C-18), anti-VAMP antibody (FL-118), anti-IP3R type II, horseradish peroxidase-conjugated donkey anti-goat IgG antibody, horseradish peroxidase-conjugated goat anti-rabbit IgG antibody and FITC-conjugated donkey anti-rabbit IgG antibody were from Santa Cruz (Santa Cruz, CA, USA). Anti-hTRPC1 polyclonal antibody was obtained from Alomone Laboratories (Jerusalem, Israel). Protein A-agarose was from Upstate Biotechnology Inc. (Madrid, Spain). Enhanced chemiluminescence detection reagents were from Pierce (Cheshire, UK). Hyperfilm ECL was from Amersham (Arlington Heights, IL, USA). All other reagents were purchased from Panreac (Barcelona, Spain).
Platelet preparation
Fura-2-loaded human platelets were prepared as previously described (Rosado et al. 2000b) as approved by Local Ethical Committees and in accordance with the Declaration of Helsinki. Briefly, blood was obtained from healthy volunteers and mixed with one-sixth volume of acid–citrate–dextrose anti-coagulant containing (mm): 85 sodium citrate, 78 citric acid and 111 d-glucose. Platelet-rich plasma was then prepared by centrifugation for 5 min at 700 g and aspirin (100 μm) and apyrase (40 μg ml−1) added. Platelet-rich plasma was incubated at 37°C with 2 μm fura-2 AM for 45 min. Cells were then collected by centrifugation at 350 g for 20 min and resuspended in Hepes-buffered saline (HBS) containing (mm): 145 NaCl, 10 Hepes, 10 d-glucose, 5 KCl, 1 MgSO4, pH 7.45 and supplemented with 0.1% w/v bovine serum albumin and 40 μg ml−1 apyrase.
Cell viability
Cell viability was assessed using calcein and trypan blue. For calcein loading, cells were incubated for 30 min with 5 μm calcein AM at 37°C and centrifuged, and the pellet was resuspended in fresh HBS. Fluorescence was recorded from 2 ml aliquots using a Shimadzu spectrophotometer (Shimadzu, Japan). Samples were excited at 494 nm and the resulting fluorescence was measured at 535 nm. After treatment with toxins for the times indicated cells were centrifuged and resuspended in fresh HBS. The calcein fluorescence remaining in the cells was the same as in control, suggesting that under our conditions there was no cellular damage. The results obtained with calcein were confirmed using the trypan blue exclusion technique. Ninety-five per cent of cells were viable after treatment with the toxins, similar to the proportion observed in our resting platelet suspensions.
Immunoprecipitation
Aliquots of 500 μl of platelet suspension (2 × 109 cells ml−1) were lysed with an equal volume of lysis buffer, pH 7.2, containing 316 mm NaCl, 20 mm Tris, 2 mm EGTA, 0.2% SDS, 2% sodium deoxycholate, 2% triton X-100, 2 mm Na3VO4, 2 mm phenylmethylsulphonyl fluoride, 100 μg ml−1 leupeptin and 10 mm benzamidine. Aliquots (1 ml) were then immunoprecipitated by incubation with 2 μg of anti-hTRPC1 polyclonal antibody or 3 μg anti-IP3R type II polyclonal antibody and protein A-agarose overnight at 4°C. Immunoprecipitates (15 μg sample−1) were resolved by 10% or 15% SDS-PAGE and Western blotting was performed as described in the following section.
Western blotting
Cell stimulation was terminated by mixing with an equal volume of 2 × Laemmli's buffer (Laemmli, 1970) with 10% dithiothreitol followed by heating for 5 min at 95°C. One-dimensional SDS-electrophoresis was performed with 10% or 15% SDS-PAGE and separated proteins were electrophoretically transferred, for 2 h at 0.8 mA cm−2, in a semi-dry blotter (Hoefer Scientific, Newcastle-under-Lyme, UK) onto nitrocellulose for subsequent probing. Blots were incubated overnight with 10% (w/v) BSA in Tris-buffered saline with 0.1% Tween 20 (TBST) to block residual protein binding sites. Blocked membranes were then incubated with the anti-SNAP-25 antibody or the anti-VAMP antibody (which recognizes three VAMP isoforms present in platelets, VAMP-1, VAMP-2 and VAMP-3 according to the manufacturer's details) diluted 1: 1500 in TBST for 1 h or anti-IP3 receptor II or anti-hTRPC1 antibody diluted 1: 500 and 1: 200, respectively, in TBST for 3 h. The primary antibody was removed and blots washed six times for 5 min each with TBST. To detect the primary antibody, blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody or horseradish peroxidase-conjugated donkey anti-goat IgG antibody, diluted 1: 10000 in TBST, washed six times in TBST, and exposed to enhanced chemiluminescence reagents for 1 min. Blots were then exposed to photographic films and the optical density was estimated using scanning densitometry.
We have previously tested the specificity of the commercial anti-hTRPC1 antibody from Alomone used in these experiments by comparison with the anti-hTRPC antibody T1E3 (Xu & Beech, 2001). After immunoprecipitation with the commercial antibody, T1E3 displayed reactivity towards exactly the same protein band (Rosado et al. 2002). To further investigate whether both antibodies recognized the same protein, both were used on the same samples. After immunoprecipitation with the commercial antibody, probing with the same antibody revealed reactivity against a protein band of approximately 100 kDa. Stripping and reprobing the blot with the T1E3 antibody revealed reactivity toward the same protein (Rosado et al. 2002). Furthermore, the commercial antibody did not detect any protein in human peripheral blood leucocytes (Rosado et al. 2002), in which hTRPC1 is reported not to be expressed (Wes et al. 1995).
Measurement of intracellular free calcium concentration ([Ca2+]i)
Fluorescence was recorded from 2 ml aliquots of magnetically stirred cell suspensions (108 cells ml−1) at 37°C using a fluorescence spectrophotometer (Varian Ltd, Madrid, Spain or Cairn Research, Faversham, UK) with excitation wavelengths of 340 and 380 nm and emission at 505 nm. Changes in [Ca2+]i were monitored using the fura-2 340/380 fluorescence ratio and calibrated according to the method of Grynkiewicz et al. (1985). Ca2+ entry in TG or thrombin-treated cells was estimated using the integral of the rise in [Ca2+]i for 2.5 minutes after addition of CaCl2 (Rosado et al. 2000b). When platelets were pre-incubated with inhibitors, Ca2+ entry was corrected by subtraction of the rise in [Ca2+]i due to leakage of the indicator. TG- and thrombin-induced Ca2+ release were estimated using the integral of the rise in [Ca2+]i for 3 min after its addition.
To compare the initial rate of Ca2+ entry between different treatments traces were fitted to the equation y = (1 − e−kt), where k is the rate constant.
Immunofluorescence
Samples of platelet suspension (200 μl; 2 × 108 cells ml−1) were transferred for 10 min to 200 μl ice-cold 3% (w/v) formaldehyde in phosphate buffered saline (PBS) containing (mm): 137 NaCl, 2.7 KCl, 5.62 Na2HPO4, 1.09 NaH2PO4, 1.47 KH2PO4 pH 7.2 and supplemented with 0.5% (w/v) bovine serum albumin. Platelets were then incubated for 1 h with 1 μg ml−1 anti-Trp1 antibody on a rocking platform. After incubation the platelets were collected by centrifugation for 1 min at 3000 g and washed twice in PBS to remove the antibody. To detect the primary antibody, samples were incubated with 0.02 μg ml−1 FITC-conjugated donkey anti-rabbit IgG antibody for 1 h and washed twice again in PBS. Fluorescence was measured using a Shimadzu fluorescence spectrophotometer (Shimadzu, Japan). Samples were excited at 496 nm and emission was at 516 nm.
Statistical analysis
Analysis of statistical significance was performed using Student's t test. For multiple comparisons, one-way analysis of variance combined with Dunnett's test was used.
Results
SNAP-25 and VAMP coimmunoprecipitate with hTRPC1 in human platelets
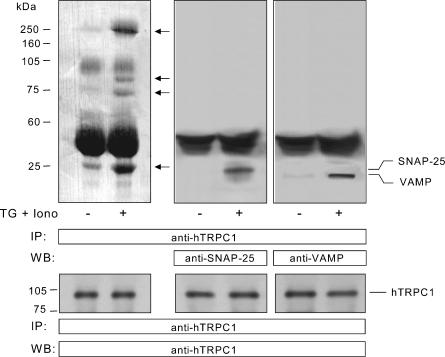
In order to investigate whether SNAP-25 and VAMPs are involved in the activation of SMCE in human platelets we tested for coupling between these proteins and hTRPC1 by looking for coimmunoprecipitation from platelet lysates. Treatment of human platelets with 1 μm TG + 50 nm Iono, to induce extensive depletion of the Ca2+ stores (Rosado & Sage, 2000b), induced the association of a number of proteins, with apparent molecular masses of 250, 90, 72 and 25 kDa (Fig. 1, top left panel), with hTRPC1 as detected by immunoprecipitation with an anti-hTRPC1 antibody followed by SDS-PAGE, transferal of proteins onto nitrocellulose membranes and staining the membranes with Ponceau stain. These proteins showed increased association with hTRPC1 after store depletion and thus might be important for the activation of SMCE. In order to investigate whether the ∼25 kDa band includes proteins of the SNARE complex, which are involved in membrane docking and fusion during exocytosis, we performed Western blotting with specific antibodies for SNAP-25 and VAMPs. After immunoprecipitation with anti-hTRPC1 antibody, Western blotting revealed the presence of SNAP-25 and VAMPs in samples from store-depleted but not control platelets (Fig. 1, top middle and right panels; n = 4). In resting platelets, coupling between hTRPC1 and SNAP-25 or VAMP was undetectable even when the amount of protein loaded in the gel was increase 2.5 times. Western blotting with anti-hTRPC1 antibody confirmed a similar content of this protein in all lanes (Fig. 1, bottom panels).
Figure 1. Coimmunoprecipitation of SNAP-25 and VAMPs with hTRP1 in human platelets.
Resting platelets and platelets treated with 1 μm TG and 50 nm Iono were lysed and whole cell lysates were immunoprecipitated with anti-hTrp1 antibody. Proteins were separated by 15% SDS-PAGE and transferred to a nitrocellulose membrane for subsequent analysis. Membranes were either stained using Ponceau stain (top left panel) or analysed by Western blotting using either anti-SNAP-25 antibody (top middle panel), anti-VAMP antibody (top right panel) or anti-hTRPC1 antibody (bottom panels) as described in Methods. Positions of molecular mass markers are shown on the left. Arrows indicate the position of immunoreactive bands which shown increased detection or are only detected in TG + Iono treated cells. These results are representative of four independent experiments performed in different donors.
Cleaving of SNAP-25 and VAMP by botulinum toxin E and tetanus toxin
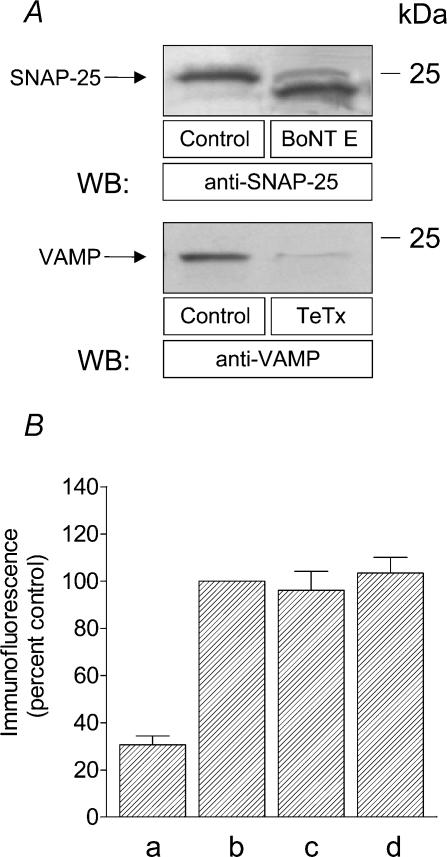
It has been previously shown that SNAP-25 and VAMP, with molecular masses of 25 and 18 kDa, respectively (Bernstein & Whiteheart, 1999), are sensitive to cleavage by the Zn2+-dependent protease activity of botulinum and tetanus toxins, respectively (Montecucco & Schiavo, 1993; Pellegrini et al. 1995). To investigate the effectiveness of treatment of human platelets with these toxins we performed a series of experiments. As shown in Fig. 2A, treatment of human platelets with botulinum toxin (BoNT) E resulted in a lower molecular weight band of SNAP-25 immunoreactivity, consistent with the removal of nine amino acids (Schiavo et al. 1993b) (Fig. 2A; n = 4). As previously described (Pellegrini et al. 1995), treatment of platelets with tetanus toxin (TeTx) resulted in complete loss of VAMP-1, -2 and -3 immunoreactivity (Fig. 2A).
Figure 2. Effect of botulinum toxin E and tetanus toxin on SNAP-25 and VAMP cleaving and surface location of hTRPC1.
A, human platelets were treated for 1 h at 37°C with 300 nm BoNT E or TeTx and lysed. Whole cell lysate proteins were separated by 15% SDS-PAGE and transferred to a nitrocellulose membrane for subsequent analysis. Membranes were analysed by Western blotting using either anti-SNAP-25 antibody (upper panel) or anti-VAMP antibody (lower panel) as described in Methods. Positions of molecular mass markers are shown on the right. These results are representative of four independent experiments performed in different donors. B, human platelets were incubated for 1 h at 37°C in the presence of 300 nm BoNT E (c), 300 nm TeTx (d) or the vehicle (b) and then fixed. The platelet suspension was then incubated with 1 μg ml−1 anti-hTrp1 antibody for 1 h followed by incubation with 0.02 μg ml−1 FITC-conjugated donkey anti-rabbit IgG antibody for a further 1 h. In (a) fixed platelets were incubated with 0.02 μg ml−1 FITC-conjugated anti-rabbit IgG antibody for 1 h. Values are mean ±s.e.m. of four independent experiments performed in different donors.
Effect of botulinum toxin E and tetanus toxin on the surface expression of hTRPC1
We have previously shown that hTRPC1 is located in the plasma membrane in platelets as detected by the anti-hTRPC1 antibody (Rosado et al. 2002), which specifically recognizes the sequence 557–551 of this protein located extracellularly between the transmembrane domains 5 and 6 (Wes et al. 1995; Zhu et al. 1995). In order to investigate whether treatment with these toxins affects the surface expression of hTRPC1 we have performed a series of immunofluorescence experiments. As shown in Fig. 2B, incubation of fixed, non-permeabilized platelets in suspension with 1 μg ml−1 anti-hTrp1 antibody followed by detection using a FITC-conjugated secondary antibody revealed the presence of hTRPC1 proteins in the platelet plasma membrane (Fig. 2B, b; n = 4), confirming the extracellular location of the epitope. The fluorescence observed cannot be attributed to non-specific binding of the secondary antibody as demonstrated by the lower fluorescence detected in samples incubated in the presence of the secondary antibody alone (Fig. 2B, a; n = 4). Treatment of human platelets with 300 nm BoNT E or TeTx for 1 h did not significantly modify the detection of hTRPC1 in the membrane (Figs 2B, c and 2 d; n = 4), which demonstrates that treatment with these toxins does not decrease the surface expression of hTRPC1 in human platelets.
Effect of botulinum toxin E on the activation of TG- and thrombin-induced SMCE
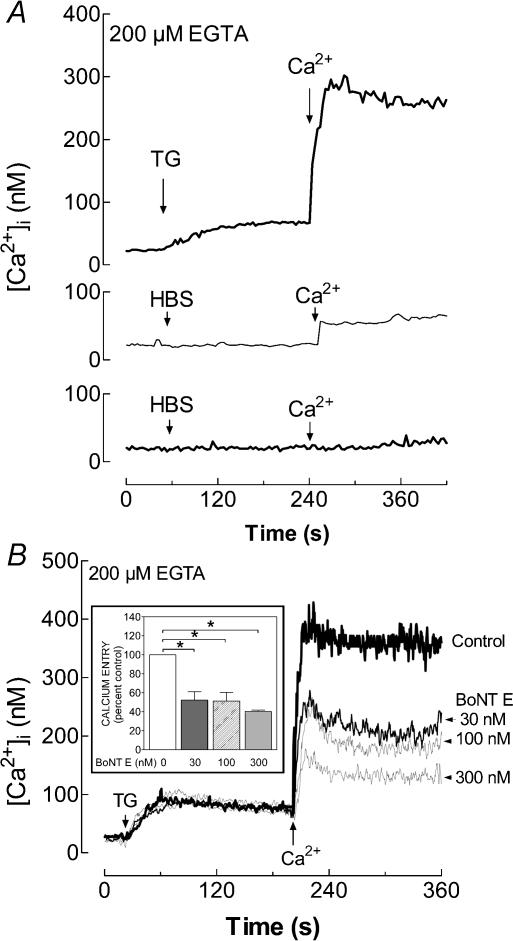
To determine whether the activity of SNAP-25 is required for the activation of SMCE we examined the effect of botulinum toxin E. In a Ca2+-free medium, TG evoked a prolonged elevation of [Ca2+]i in platelets due to release of Ca2+ from intracellular pools. Subsequent addition of Ca2+ (300 μm) to the external medium induced a sustained increase in [Ca2+]i, indicative of SMCE (Fig. 3A (top panel) and B). In resting platelets incubated for 1 h at 37°C, Ca2+ addition induced a small but sustained increase in [Ca2+]i (Fig. 3A, middle panel), which might be attributed to fura-2 leakage due to the temperature of the incubation since this effect was not observed in resting platelets maintained for 1 h at room temperature (Fig. 3A, bottom panel). Therefore, when cells were pre-incubated with inhibitors, Ca2+ entry was corrected by subtraction of the rise in [Ca2+]i due to leakage of the indicator.
Figure 3. Effect of botulinum toxin E on the activation of TG-evoked Ca2+ entry in human platelets.
A, resting fura-2-loaded platelets (top and bottom panels) or platelets incubated for 1 h at 37°C (middle panel) were stimulated with TG (1 μm; top panel) or the vehicle (HBS; middle and bottom panels), as indicated, in a Ca2+-free medium (200 μm EGTA was added). CaCl2 (final concentration 300 μm) was added to the medium 3 min later. Elevations in [Ca2+]i were monitored using the 340/380 nm ratio and traces were calibrated in terms of [Ca2+]i. B, platelets were incubated for 1 h with several concentrations of BoNT E (30–300 nm) at 37°C and then stimulated with TG (1 μm) in a Ca2+-free medium (200 μm EGTA was added). CaCl2 (final concentration 300 μm) was added to the medium 3 min later to initiate Ca2+ entry. Data are presented as mean values of 4–12 experiments. Inset: bar graph showing the Ca2+ entry under different experimental conditions expressed as the percentage of control. Values are mean ±s.e.m. of 4–12 experiments performed using six different donors. *P < 0.01 compared to control.
Pretreatment of human platelets for 1 h at 37°C with BoNT E decreased SMCE in a concentration-dependent manner (Fig. 3B; P < 0.01; n = 4–12). The initial rates of Ca2+ entry were 0.306 ± 0.011 nm s−1 in control platelets and 0.137 ± 0.007, 0.120 ± 0.007 and 0.109 ± 0.005 nm s−1 in platelets treated with 30, 100 and 300 nm BoNT E, respectively. Preincubation with BoNT E did not modify the TG-induced Ca2+ release (Fig. 3B), suggesting that this treatment did not affect the ability of platelets to store Ca2+ in intracellular compartments. These results suggest that SNAP-25 is involved in the activation of SMCE in human platelets.
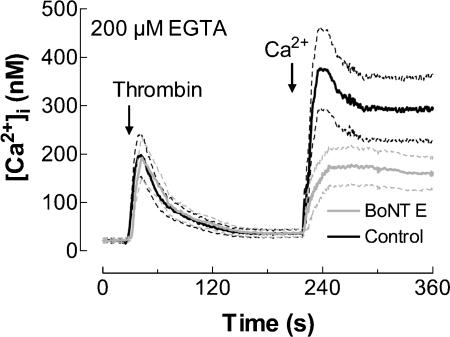
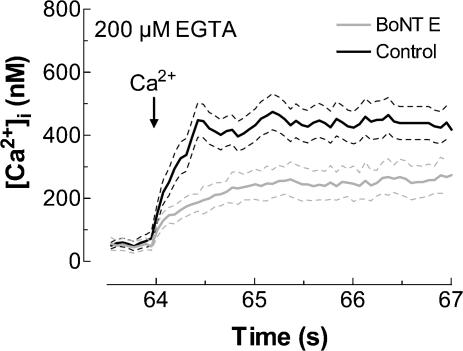
We further investigated the involvement of SNAP-25 in SMCE evoked by the physiological agonist thrombin. Treatment of human platelets with thrombin (1 U ml−1) induced a transient increase in [Ca2+]i due to release from intracellular stores (Fig. 4). Pretreatment of platelets with 300 nm BoNT E for 1 h at 37°C significantly decreased SMCE (Fig. 4; P < 0.01; n = 5). The initial rates of Ca2+ entry were 0.191 ± 0.010 and 0.119 ± 0.002 nm s−1 in the absence and presence of BoNT E, respectively. Treatment with BoNT E did not modify thrombin-evoked release of Ca2+ from stores, which confirms that this treatment did not affect the ability of platelets to store Ca2+ in intracellular pools. In addition, these findings indicate that BoNT E does not interfere with the agonist-activated intracellular release pathways and that it does not alter the membrane receptors for thrombin.
Figure 4. Effect of botulinum toxin E on the activation of thrombin-induced Ca2+ entry in human platelets.
Fura-2-loaded human platelets were incubated for 1 h in the presence of 300 nm BoNT E at 37°C and then stimulated with thrombin (1 U ml−1), as indicated, in a Ca2+-free medium (200 μm EGTA was added). CaCl2 (final concentration 300 μm) was added to the medium at the time indicated to initiate Ca2+ entry. Elevations in [Ca2+]i were monitored using the 340/380 nm ratio and traces were calibrated in terms of [Ca2+]i. Data are presented as mean ±s.e.m. of five experiments performed using five different donors.
Effect of botulinum toxin E on the maintenance of SMCE
SNAREs, such as SNAP-25, have been shown to participate in the machinery that leads to membrane fusion or the establishment of membrane contacts (Duman & Forte, 2003). The latter is proposed to form the basis of the conformational coupling model for the activation of SMCE. However, for the maintenance of Ca2+ entry an actin cytoskeleton based scaffold has been shown to be required (Rosado et al. 2000b). To investigate the involvement of SNAP-25 in the maintenance of SMCE we examined the effect of botulinum toxin on Ca2+ entry once SMCE had been activated using TG. Figure 5 shows the effect of the addition of BoNT E to store-depleted human platelets. Botulinum toxin at 300 nm or the vehicle was added to platelets 3 min after TG. As shown in Fig. 3 at the time when the toxin was added Ca2+ entry had already been activated. Cells were then incubated for a further 1 h before the addition of Ca2+ to the medium to initiate Ca2+ entry. Addition of BoNT E after activation of SMCE significantly reduced the maintenance of Ca2+ entry in platelets (Fig. 5; P < 0.01; n = 5). The initial rates of Ca2+ entry were 0.150 ± 0.009 and 0.059 ± 0.003 nm s−1 in the absence and presence of BoNT E, respectively. These observations suggest a role for SNAP-25 both in the activation and the maintenance of SMCE in these cells.
Figure 5. Effect of botulinum toxin E on the maintenance of TG-induced Ca2+ entry in human platelets.
Fura-2-loaded human platelets were suspended in a Ca2+-free medium (200 μm EGTA added). Cells were then stimulated with TG (1 μm) and 3 min later 300 nm BoNT E or the vehicle (HBS; control) was added. CaCl2 (final concentration 300 μm) was added to the medium 1 h after the addition of the toxin or the vehicle to initiate Ca2+ entry. Data are presented as mean ±s.e.m. of five experiments performed in five different donors.
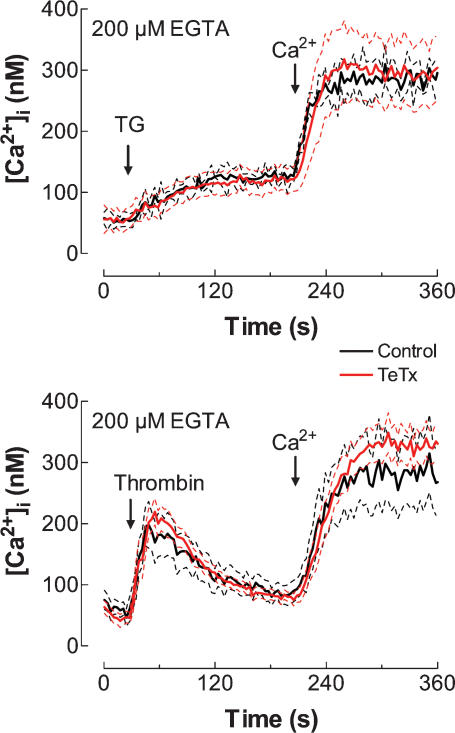
Effect of tetanus toxin on the activation of TG- and thrombin-induced SMCE
Human platelets have been reported to express several VAMP isoforms, including VAMP-1, -2 and -3 (Bernstein & Whiteheart, 1999; Polgar et al. 2002). We next investigated the possible role of VAMPs in SMCE induced by either TG or thrombin in human platelets. Human platelets were pre-incubated for 1 h at 37°C with 300 nm TeTx, a treatment that, as shown in Fig. 2, induces massive cleavage of VAMPs in platelets. However, despite the established VAMP cleavage, pre-incubation of human platelets with TeTx did not alter either TG- or thrombin-induced Ca2+ entry (Fig. 6; n = 11–17). As with botulinum toxin, treatment with tetanus toxin did not modify TG- or thrombin-induced Ca2+ release, which indicates that this treatment did not affect the ability of platelets to store Ca2+ or interfere with the Ca2+ release mechanisms activated by these agents.
Figure 6. Effect of tetanus toxin on the activation of TG- or thrombin-induced Ca2+ entry in human platelets.
Fura-2-loaded platelets were incubated for 1 h in the presence of TeTx (300 nm) at 37°C and then stimulated with TG (1 μm) or thrombin (1 U ml−1), as indicated, in a Ca2+-free medium (200 μm EGTA was added). CaCl2 (final concentration 300 μm) was added to the medium at the time indicated to initiate Ca2+ entry. Elevations in [Ca2+]i were monitored using the 340/380 nm ratio and traces were calibrated in terms of [Ca2+]i. Data are presented as mean ±s.e.m. of 17 experiments performed in 10 different donors.
Botulinum toxin E reduces the coupling between hTRPC1 and IP3 receptor type II in human platelets
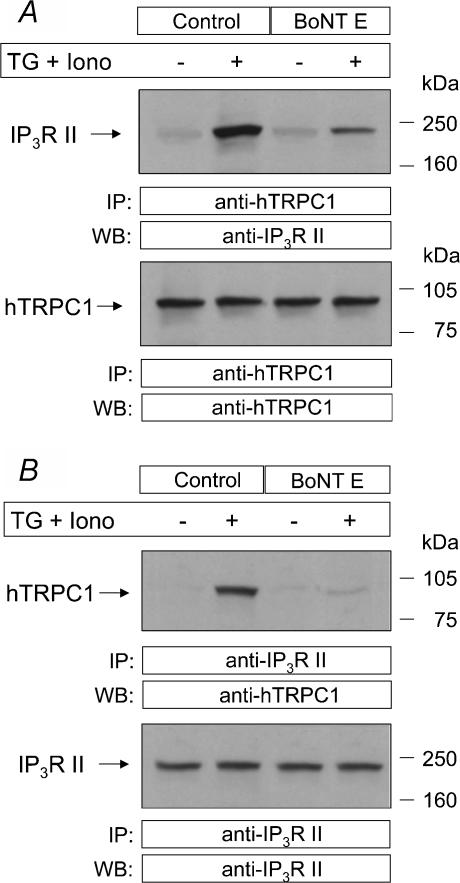
We have previously reported that depletion of the intracellular Ca2+ stores stimulates a de novo and rapid coupling between endogenously expressed hTRPC1 and the type II IP3R in human platelets independently of rises in [Ca2+]i, a process that might account for the activation of SMCE in these cells (Rosado & Sage, 2000b; Brownlow & Sage, 2003). Since BoNT E inhibited SMCE in platelets, we have investigated the effect of this toxin on the coupling between hTRPC1 and type II IP3R. Consistent with our previous studies (Rosado & Sage, 2000b; Brownlow & Sage, 2003) no coupling was found in resting cells while it was clearly detectable after treatment with 1 μm TG + 50 nm Iono to strongly deplete the intracellular stores (Fig. 7). In agreement with the effect of BoNT E on SMCE, treatment of human platelets for 1 h with 300 nm BoNT E reduced the coupling between hTRPC1 and IP3R type II by 70%(P < 0.01; n = 3). Our results also indicate that treatment. with BoNT E does not modify the amount of hTRPC1 or IP3 R II detectable in human platelets (Fig. 7).
Figure 7. BoNT E reduces the coupling between hTRPC1 and the type II IP3R in store-depleted human platelets.
Human platelets were pre-incubated in the absence or presence of BoNT E (300 nm) for 1 h at 37°C. Cells were then stimulated with TG (1 μm) plus Iono (50 nm) and then lysed. A, whole cell lysates were immunoprecipitated (i.p.) with anti-hTRPC1 antibody and analysed by Western blotting (WB) using anti-IP3R type II antibody (anti-IP3R II; top panel) or anti-hTRPC1 antibody (bottom panel). B, whole cell lysates were immunoprecipitated (i.p.) with anti-IP3R II antibody and analysed by Western blotting (WB) using anti-hTRPC1 antibody (top panel) or anti-IP3R II antibody (bottom panel). Positions of molecular mass markers are shown on the right. These results are representative of three independent experiments performed using different donors.
Discussion
The SNARE superfamily of proteins has become one of the most extensively studied elements of the mechanism involved in intracellular trafficking. The roles of these proteins in membrane trafficking, docking and fusion include the establishment of tight membrane contacts and the formation of a scaffold to support the secretory machinery (Duman & Forte, 2003). Among the most important and ubiquitous SNARE proteins are VAMPs, transmembrane proteins located in the vesicular membranes, and SNAP-25, which contains a region of palmitoylated cysteines by which it associates with the plasma membrane (Lin & Scheller, 2000). Since the activation of SMCE has been shown to share properties with the secretory process in several non-excitable cells, including platelets (Rosado et al. 2000b), we have investigated whether SNAP-25 and VAMP are necessary for SMCE in these cells.
We have previously shown that hTRPC1 and the type II IP3R couple upon store depletion in platelets (Rosado & Sage, 2000b), a process modulated by the actin cytoskeleton, which exerts both facilitating and inhibitory roles in this process (Rosado & Sage, 2001a), as in secretion (Muallem et al. 1995). We have also provided evidence that both functional IP3Rs and hTRPC1 are required for SMCE in these cells (Rosado & Sage, 2000b; Rosado et al. 2002), and that the de novo coupling between these proteins occurs rapidly enough to account for agonist-evoked SMCE (Brownlow & Sage, 2003). Human platelets are reported to express SNAP-25 and VAMPs (Bernstein & Whiteheart, 1999; Reed et al. 1999; Polgar et al. 2002). We therefore tested for coupling between these SNARE proteins and hTRPC1 by looking for coimmunoprecipitation from platelet lysates. As previously reported for the coupling between hTRPC1 and the type II IP3 receptor (Rosado & Sage, 2000b), after immunoprecipitation with anti-hTRPC1 antibody, Western blotting revealed the presence of SNAP-25 and VAMP in samples from store-depleted but not control platelets. These findings provide evidence that store depletion induces the formation of a protein complex, including hTRPC1, the type II IP3R, SNAP-25 and VAMPs.
The functional involvement of SNARE in SMCE was investigated using the highly specific botulinum and tetanus toxins, which cleave SNAP-25 and VAMPs, respectively (Schiavo et al. 1992, 1993b). BoNT E has been shown to remove a 3 kDa peptide from the carboxyl-terminus of SNAP-25 yielding a product of 20.5 kDa (Schiavo et al. 1993a), which accounts for the reduction of size of this protein after treatment with BoNT E as seen in Fig. 2. Cleavage of VAMPs by TeTx is more centrally targeted and produces two small fragments that are undetectable with the gel resolution used.
Preincubation of platelets with BoNT E reduced SMCE evoked either by TG or thrombin in a concentration-dependent manner. However, we found that complete cleavage of SNAP-25 reduced Ca2+ entry only by 60%, suggesting that this protein is only partially required for the activation of SMCE in these cells. Our results clearly show that BoNT E inhibited TG- or thrombin-induced SMCE without having any effect on the release of Ca2+ from the intracellular stores, which suggests that the effect of this toxin occurs entirely through the modulation of Ca2+ entry. Our findings are consistent with those for Xenopus oocytes (Yao et al. 1999). Furthermore, addition of botulinum toxins to platelet suspensions after the activation of SMCE partially reversed Ca2+ entry already activated by TG suggesting that SNAP-25 is partially required both for the activation and for the maintenance of SMCE in human platelets. The effects of BoNT E on SMCE cannot be attributed to a decrease in the surface expression of hTRPC1 as demonstrated by immunofluorescence techniques. In contrast to the results with SNAP-25, we found using TeTx that VAMPs are not required for the activation of SMCE in human platelets.
A number of studies in different cell types have reported that multiple mechanisms can regulate the activation of SMCE. Conformational coupling is the model that best describes the mechanism of activation of SMCE in several cell types, such as platelets (Rosado et al. 2000a,b; Rosado & Sage, 2000a), pancreatic acinar cells (Redondo et al. 2003), smooth muscle cells (Patterson et al. 1999; Yao et al. 1999), human pulmonary arterial endothelial cells (HPAEC; Mehta et al. 2003), glioma C6 cells (Sabala et al. 2002) and the human hepatocellular carcinoma cell line HepG2 (Rosado et al. 2001). However, in other cells, such as vascular smooth muscle cells (Smani et al. 2004), RBL-1 cells (Bakowski et al. 2001; Bakowski et al. 2003) or Xenopus oocytes (Thomas et al. 1996), the conformational coupling model appears not to be responsible for the activation of SMCE and an alternative mechanism, such as the release of a diffusible molecule upon store depletion, might account for this process in these cells. In the conformational coupling mechanism the remodelling of the actin cytoskeleton plays an important role, but depending on the cell type the contribution of this to SMCE is more or less relevant. While in vascular endothelial cells (Holda & Blatter, 1997), HPAEC (Mehta et al. 2003), glioma C6 cells (Sabala et al. 2002) and the human hepatocellular carcinoma cell line HepG2 (Rosado et al. 2001) inhibition of actin polymerization by cytochalasin D or latrunculin A completely disorganizes the actin network and fully blocks SMCE, in human platelets, where actin treadmilling is very slow (Fox & Phillips, 1981), these agents prevent actin polymerization without modifying the resting actin network and only reduce SMCE by about 50% (Rosado et al. 2000b; Rosado & Sage, 2000a). A similar partial effect was found in pancreatic acinar cells (Redondo et al. 2003). We have recently demonstrated in platelets the coexistence of a cytoskeleton-independent pathway activating SMCE that is mediated by extracellular signal-regulated kinases 1 and 2 (ERK1/2) (Rosado & Sage, 2001b). Since combined inhibition of actin polymerization and ERK1/2 resulted in almost complete inhibition of SMCE, the ERK1/2 pathway appears to be responsible for the activation of most of the cytoskeleton-independent SMCE. Our results suggest that SNAP-25 is involved in about 50% of the SMCE response. These proteins might be components of the cytoskeleton-dependent or -independent branch of the cellular machinery for the activation of Ca2+ entry, although it is more likely that SNAP-25 belongs to the cytoskeleton-dependent pathway since vesicle trafficking and fusion require the support of the actin filament network. In line with our observations in platelets, Yao et al. (1999) found that BoNT A only partially reduced SMCE in Xenopus oocytes.
Since SNAP-25 is required for the activation and maintenance of SMCE in platelets, where a de novo coupling between naturally expressed hTRPC1 and the IP3 receptor type II has been demonstrated (Rosado & Sage, 2000b), we have investigated its involvement in this coupling. Our results indicate that functional SNAP-25 is involved in the coupling between hTRPC1 and the type II IP3R in human platelets. As previously reported (Rosado & Sage, 2001a; Brownlow & Sage, 2003), the remarkable correlation between the characteristics of the coupling and the activation of SMCE support the hypothesis that coupling between hTRPC1 and type II IP3R underlies the activation of SMCE in these cells.
Our results suggest that in human platelets SNAP-25 is involved in a protein complex, induced by Ca2+ store depletion, that could lead to the establishment of a tight contact between the membranes of the ER and PM in order to facilitate the coupling between Ca2+ channels in the PM and IP3 receptors in the ER. In addition, SNAP-25 seems to be required to maintain Ca2+ entry once the stores are depleted, perhaps as a component of the scaffold that supports the close apposition of the membranes. In platelets, the regulation of SMCE by SNARE does not seem to involve VAMPs or involves a TeTx-insensitive VAMP isoform (Galli et al. 1998).
Our observations shed new light on the mechanisms involved in SMCE in human platelets. The data presented are consistent with the existence of a conformational coupling mechanism to account for the activation and maintenance of SMCE in these cells. On the basis of this model, store depletion by agonists might induce the activation of a machinery for trafficking of portions of the ER towards the PM, not for fusion, but to facilitate the coupling between Ca2+ channels in the PM and IP3 receptors in the ER where SNAP-25 might play a key role.
Acknowledgments
We thank Mercedes Gómez Blázquez for her technical assistance and James Diver, Matthew Harper and Stephen Key for participation in preliminary experiments. This work was supported by MCyT-DGI grant BFI2001-0624, the Wellcome Trust (grant no. 064070) and Junta de Extremadura-Consejería de Sanidad y Consumo grant 03/51. P.C.R. is supported by a DGESIC fellowship (BFI2001-0624). A.G.S.H. held an Undergraduate Summer Bursary from the Nuffield Foundation.
References
- Bakowski D, Burgoyne RD, Parekh AB. Activation of the store-operated calcium current ICRAC can be dissociated from regulated exocytosis in rat basophilic leukaemia (RBL-1) cells. J Physiol. 2003;553:387–393. doi: 10.1113/jphysiol.2003.055335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski D, Glitsch MD, Parekh AB. An examination of the secretion-like coupling model for the activation of the Ca2+ release-activated Ca2+ current ICRAC in RBL-1 cells. J Physiol. 2001;532:55–71. doi: 10.1111/j.1469-7793.2001.0055g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein AM, Whiteheart SW. Identification of a cellubrevin/vesicle associated membrane protein 3 homologue in human platelets. Blood. 1999;93:571–579. [PubMed] [Google Scholar]
- Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc Natl Acad Sci U S A. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow S, Sage SO. Rapid agonist-evoked coupling of type II Ins(1,4,5)P3 receptor with human transient receptor potential (hTRPC1) channels in human platelets. Biochem J. 2003;375:697–704. doi: 10.1042/BJ20030929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman JG, Forte JG. What is the role of SNARE proteins in membrane fusion? Am J Physiol Cell Physiol. 2003;285:237–249. doi: 10.1152/ajpcell.00091.2003. [DOI] [PubMed] [Google Scholar]
- Fox JEB, Phillips DR. Inhibition of actin polymerization in blood platelets by cytochalasins. Nature. 1981;292:650–652. doi: 10.1038/292650a0. [DOI] [PubMed] [Google Scholar]
- Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Holda JR, Blatter LA. Capacitative calcium entry is inhibited in vascular endothelial cells by disruption of cytoskeletal microfilaments. FEBS Lett. 1997;403:191–196. doi: 10.1016/s0014-5793(97)00051-3. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin RC, Scheller RH. Mechanisms of synaptic vesicle exocytosis. Annu Rev Cell Dev Biol. 2000;16:19–49. doi: 10.1146/annurev.cellbio.16.1.19. [DOI] [PubMed] [Google Scholar]
- Liu XB, Wang WC, Singh BB, Lockwich T, Jadlowiec J, O'Connell B, Wellner R, Zhu MX, Ambudkar IS. Trp1, a candidate protein for the store-operated Ca2+ influx mechanism in salivary gland cells. J Biol Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2s+ entry. Role in signaling increased endothelial permeability. J Biol Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Schiavo G. Tetanus and botulism neurotoxins: a new group of zinc proteases. Trends Biochem Sci. 1993;18:324–327. doi: 10.1016/0968-0004(93)90065-u. [DOI] [PubMed] [Google Scholar]
- Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Gill DL. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- Pellegrini LL, O'Connor V, Lottspeich F, Betz H. Clostridial neurotoxins compromise the stability of a low energy SNARE complex mediating NSF activation of synaptic vesicle fusion. EMBO J. 1995;14:4705–4713. doi: 10.1002/j.1460-2075.1995.tb00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar J, Chung SH, Reed GL. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood. 2002;100:1081–1083. doi: 10.1182/blood.v100.3.1081. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr TRP, inositol 1,4,5-trisphosphate receptors, and capacitative calcium entry. Proc Natl Acad Sci U S A. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW, Jr, McKay RR. Capacitative calcium entry channels. Bioessays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Redondo PC, Lajas AI, Salido GM, González A, Rosado JA, Pariente JA. Evidence for secretion-like coupling involving pp60src in the activation and maintenance of store-mediated Ca2+ entry in mouse pancreatic acinar cells. Biochem J. 2003;370:255–263. doi: 10.1042/BJ20021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed GL, Aiilyan KH, Fitzgerald ML. Human platelets contain SNARE proteins and a Sec1p homologue that interacts with syntaxin 4 and is phosphorylated after thrombin activation: implications for platelet secretion. Blood. 1999;93:2617–2626. [PubMed] [Google Scholar]
- Rink TJ, Sage SO. Calcium signaling in human platelets. Annu Rev Physiol. 1990;52:431–439. doi: 10.1146/annurev.ph.52.030190.002243. [DOI] [PubMed] [Google Scholar]
- Rosado JA, Brownlow SL, Sage SO. Endogenously expressed Trp1 is involved in store-mediated Ca2+ entry by conformational coupling in human platelets. J Biol Chem. 2002;277:42157–42163. doi: 10.1074/jbc.M207320200. [DOI] [PubMed] [Google Scholar]
- Rosado JA, Graves D, Sage SO. Tyrosine kinases activate store-mediated Ca2+ entry in human platelets through the reorganization of the actin cytoskeleton. Biochem J. 2000a;351:429–437. [PMC free article] [PubMed] [Google Scholar]
- Rosado JA, Jenner S, Sage SO. A role for the actin cytoskeleton in the initiation and maintenance of store-mediated calcium entry in human platelets. Evidence for conformational coupling. J Biol Chem. 2000b;275:7527–7533. doi: 10.1074/jbc.275.11.7527. [DOI] [PubMed] [Google Scholar]
- Rosado JA, Rosenzweig I, Harding S, Sage SO. Tumor necrosis factor-alpha inhibits store-mediated Ca2+ entry in the human hepatocellular carcinoma cell line HepG2. Am J Physiol Cell Physiol. 2001;280:1636–1644. doi: 10.1152/ajpcell.2001.280.6.C1636. [DOI] [PubMed] [Google Scholar]
- Rosado JA, Sage SO. Farnesylcysteine analogues inhibit store-regulated Ca2+ entry in human platelets: evidence for involvement of small GTP-binding proteins and actin cytoskeleton. Biochem J. 2000a;347:183–192. [PMC free article] [PubMed] [Google Scholar]
- Rosado JA, Sage SO. Coupling between inositol 1,4,5-trisphosphate receptors and human transient receptor potential channel 1 when intracellular Ca2+ stores are depleted. Biochem J. 2000b;350:631–635. [PMC free article] [PubMed] [Google Scholar]
- Rosado JA, Sage SO. Activation of store-mediated calcium entry by secretion-like coupling between the inositol 1,4,5-trisphosphate receptor type II and human transient receptor potential (hTrp1) channels in human platelets. Biochem J. 2001a;356:191–198. doi: 10.1042/0264-6021:3560191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado JA, Sage SO. Role of the ERK pathway in the activation of store-mediated calcium entry in human platelets. J Biol Chem. 2001b;276:15659–15665. doi: 10.1074/jbc.M009218200. [DOI] [PubMed] [Google Scholar]
- Sabala P, Targos B, Caravelli A, Czajkowski R, Lim D, Gragnaniello G, Santella L, Baranska J. Role of the actin cytoskeleton in store-mediated calcium entry in glioma C6 cells. Biochem Biophys Res Commun. 2002;296:484–491. doi: 10.1016/s0006-291x(02)00893-8. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Rossetto O, Catsicas S, Polverino de Laureto P, DasGupta BR, Benfenati F, Montecucco C. Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J Biol Chem. 1993a;268:23784–23787. [PubMed] [Google Scholar]
- Schiavo G, Santucci A, Dasgupta BR, Mehta PP, Jontes J, Benfenati F, Wilson MC, Montecucco C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993b;335:99–103. doi: 10.1016/0014-5793(93)80448-4. [DOI] [PubMed] [Google Scholar]
- Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Thomas D, Kim HY, Hanley MR. Regulation of inositol trisphosphate-induced membrane currents in Xenopus oocytes by a Jurkat cell calcium influx factor. Biochem J. 1996;318:649–656. doi: 10.1042/bj3180649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- Yao Y, Ferrer-Montiel AV, Montal M, Tsien RY. Activation of store-operated Ca2+ current in Xenopus oocytes requires SNAP-25 but not a diffusible messenger. Cell. 1999;98:475–485. doi: 10.1016/s0092-8674(00)81976-5. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]