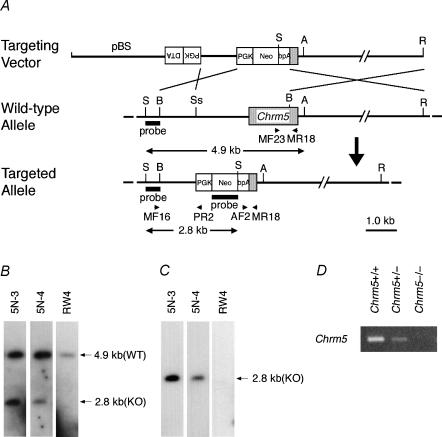
Figure 1. Generation of Chrm5-deficient mice.
A, targeting strategy by homologous recombination in ES cells. The targeting vector pChrm5-N2 contained the neo gene and the diphtheria toxin α-subunit gene (DTA) driven by the phosphoglycerate kinase I promoter (PGK). The arrowheads marked MF16 and PR2 indicate the PCR primers used for homologous recombinant screening, and those marked MF23, AF2 and MR18 indicate the primers used for genotyping. ApaI (A), BamHI (B), HindIII, EcoRI (R), SacI (S), and SspI (Ss) sites relevant to the identification of homologous recombinant ES cell clones are shown, as well as the expected size of the bands hybridizing with the Chrm5 and neo probes (indicated by 4.9 and 2.8 kb bands and arrows). B and C, confirmation of homologous recombination in ES cell clones by Southern hybridization. B, hybridization with the Chrm5 probe showing the 2.8 kb band specific for the targeted allele and the 4.9 kb band derived from the wild-type allele. 5N-3 and 5N-4 are representative homologous recombinant clones, whereas RW4 is a parental ES cell line. C, hybridization with the neo probe showing the 2.8 kb band specific for the targeted allele. D, RT-PCR analysis showing Chrm5 mRNA levels in the brains of the wild-type and heterozygous and homozygous mutant mice. The levels were lower in the heterozygous brain than in the wild-type brain. No PCR product was seen with the homozygous mutant.