Abstract
The potential of FDG-PET and MRI in monitoring response to treatment in lymphoma is reviewed. Both FDG-PET and MRI can provide whole body imaging. Both also share the advantage of combining functional and anatomical information. At present, hybrid FDG-PET and MDCT is the best technique for monitoring response to treatment, especially early response to treatment. Early assessment of response to treatment has the potential to tailor therapy. MR imaging is useful especially in assessing bone marrow and central nervous system involvement.
Keywords: MRI, FDG-PET, monitoring response, lymphoma
Introduction
So-called ‘functional imaging’ has been used in the evaluation of lymphoma to supplement the information obtained from computed tomography (CT). The main limitations of CT-based International Workshop Criteria (ICW) are: (1) the limited accuracy of CT at initial staging for assessing lymphoma in small nodes (<1 to 1.5 cm), bone marrow, or various extranodal sites; (2) the inability of CT to differentiate active disease within a residual mass; and (3) the limited ability of CT to assess early response to treatment although more aggressive, but also potentially more toxic treatments are now available [1]. CT, however, remains the method of choice for initial measurements of involved sites and detection of complications such as adjacent organ compression. During follow-up, CT monitors size changes, and is useful for diagnosing treatment complications. Moreover, CT can also be seen as a potential ‘functional’ imaging. Dugdale et al. showed that perfusion values measured at CT decrease when lymphoma masses become inactive (Fig. 1) [2]. To date, however, no study has confirmed these preliminary data. Other functional imaging tools are used for the evaluation of residual masses. Gallium imaging is not an accurate technique for detecting sites of involvement at diagnosis, with frequent false-negative results compared to CT, and also false-positive para-hilar uptake [3]. However, when an initial nodal site is gallium avid at diagnosis, follow-up gallium scans assess tumour activity during treatment. Janicek et al. demonstrated that early restaging gallium scans delineate patients who are likely to have prolonged disease-free survival from those who fail to respond to intensive therapy [4]. Patients whose tumours remain Ga-positive midway through chemotherapy have a poor outcome [4]. However, gallium imaging is a complex technique with several disadvantages when compared to [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) including: lower contrast and resolution, the need to perform acquisition 48 h after gallium citrate injection, higher dosimetry, and longer scanning time. Furthermore, gallium binds to plasma transferrin after which it binds to receptors on the surface of lymphoma cells leading to potentially false negative results. FDG uptake reflects a general metabolic process common to most malignant tissues with therefore probably less false negative results. The objective of this paper is to review the potential of FDG-PET and magnetic resonance imaging (MRI) in monitoring response to treatment in lymphoma.
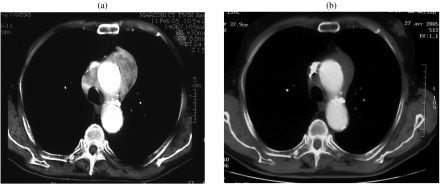
Figure 1.
A 78-year-old patient with mediastinal NHL. (a) Enhanced baseline CT scan showed an enhancing 7×4 cm large mediastinal mass. (b) After three cycles of chemotherapy, enhanced CT scan of the mediastinum demonstrated partial response, but with decreased enhancement of the residual mass. After completion of treatment, the patient was considered in complete response (uncertain).
FDG-PET
Briefly, FDG is transported into viable cells by glucose transporter molecules, where it is phosphorylated by hexokinase into FDG-6-phosphate, just as glucose is normally phosphorylated into glucose-6-phosphate. Unlike glucose-6-phosphate, however, FDG-6-phosphate undergoes no further metabolism within the cell. Moreover, its dephosphorylation by glucose-6-phosphatase is a relatively slow process in comparison to that of glucose-6-phosphatase. This, combined with the fact that FDG-6-phosphate cannot easily cross the cell membrane, results in entrapment of FDG-6-phosphate within viable cells. Malignant cells have an increased rate of aerobic glycolysis, compared to normal tissue. They also have a greater number of glucose transport molecules at the cell surface and a lower level of glucose-6-phosphatase.
Fluorine-18 is a positron emitter. The emitted positron penetrates only a few millimetres into tissues before combining with an electron. The particle pair then annihilates and its mass is entirely converted into energy. This energy takes the form of two 511 keV annihilation photons, emitted at approximately 180^ from each other. Detection of both photons is termed coincidence detection, and this is the principle by which PET operates. Fluorine-18 has a half-life of 110 min, allowing acquisition of images over 30–120 min.
The biodistribution of FDG can be affected by various physiologic factors [5]. Blood glucose levels have an impact on FDG uptake through (a) competitive displacement of FDG by plasma glucose, and (b) patients being asked to fast for 6 h prior to imaging. Good control of blood glucose is essential; a level of less than 150 mg /dl is desirable. Because the primary route of FDG excretion is renal, good hydration is required. Muscle relaxants may be used to reduce muscle uptake. Patients are asked to remain silent after injection. The usual dose of FDG is 10–15 mCi. To our knowledge, there is no contra-indication for FDG administration. PET imaging is initiated approximately 60 min following the injection of FDG.
Hybrid FDG-PET/CT
Hybrid PET/CT scanners combine a PET and a CT machine housed back-to-back. This enables image acquisition in the same position with PET and CT, thus enabling the precise combination of the anatomic information provided by CT and the functional data provided by FDG-PET. Although most centres acquire unenhanced CT images, other centres perform single phase enhanced CT with orally administered water-soluble iodinated contrast media [6]. This could allow optimal CT images as multidetector CT (MDCT) is now standard in new hybrid PET/CT devices.
Monitoring response to treatment with FDG-PET
Staging of lymphoma
The accuracy of FDG-PET as an imaging tool for primary staging of lymphoma suffers from the absence of a systematic pathological correlation (Fig. 2). In our experience, PET alone is concordant with conventional imaging and bone marrow biopsy (BMB) in only 80% of cases [7], better than conventional imaging and BMB in 8% and worse in 12%. Among these latter cases, one-third account for bone marrow involvement undetected by PET. Other studies have reported similar results [8]. Moog et al. showed that FDG-PET was more accurate for detecting nodal lymphoma than CT [8]. Seven lymph node regions unremarkable on conventional CT showed increased uptake of FDG. Staging was changed in the 4/60 patients with these seven confirmed additional PET findings: from stage I to II in one patient, and from stage II to III in three patients. The clinically relevant question of how PET impacts on the staging of lymphoma and above all, whether or not up- or down-staging leads to changes in therapeutic strategies, has been addressed in some studies with variable results. For Shöder et al. PET-FDG could contribute to changes in clinical stage in 44% and changes in treatment in more than 60% of cases [9].
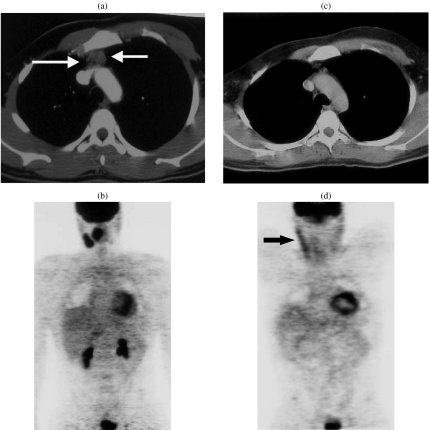
Figure 2.
A 41-year-old patient presenting with cervical enlarged lymph nodes with NHL involvement. (a) CT scan of the upper mediastinum showed an anterior mediastinal mass (arrows), without FDG uptake on PET. (b) This mass could correspond to an enlarged thymus. (c) Post-treatment CT scan performed 3 months later showed that the anterior mass was no longer present. (d) FDG-PET was normal; the linear cervical uptake (arrow) corresponded to physiologic muscle uptake. This case demonstrates the potential discordances between CT and FDG-PET due to the absence of pathological examination of the anterior mediastinal mass.
FDG-PET: significance of positive findings
Several limitations of FDG-PET have been reported: physiologic muscles may take up the radiotracer and show increased activity on the PET images (Fig. 2) [6]; This muscle uptake is easily identified when compared to CT images. Similarly, physiologic uptake by the kidneys, bowel and liver can be distinguished on combined CT images. Physiologic FDG uptake has also been reported in brown fat [10]. Other false-positive findings of FDG-PET, more difficult to recognize, include inflammatory changes caused by infectious or inflammatory processes such as viral infections, bronchitis, aspergilloma, sarcoidosis, etc. [10, 11]
Response to treatment
PET is able to distinguish between active tumour and inactive residual masses often present following treatment (Fig. 3) [12–14]. This use is probably the most important especially in aggressive NHL and Hodgkin’s lymphoma. False-positive findings at the site of residual masses may be seen, however, due to rebound thymic hyperplasia or post-therapy inflammatory changes especially following radiotherapy as well as infectious or inflammatory processes outside the site of residual masses [14]. The diffusely increased bone marrow uptake often observed during treatment and related to the administration of growth factor is usually linked to bone marrow hyperplasia and should not be misinterpreted as specific involvement (Fig. 3). FDG-PET results after treatment can predict therapy outcomes [15]. However, a negative PET scan cannot rule out the presence of minimal residual disease [5].
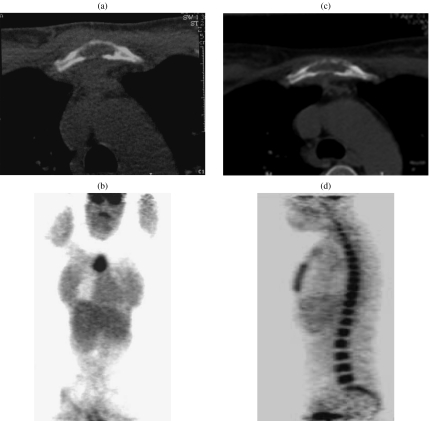
Figure 3.
A 25-year-old patient with NHL presenting with a soft tissue sternal mass associated with lytic sternal bone on CT (a) and increased isolated uptake on FDG-PET (b) concordant with CT findings. (c) After treatment, CT showed decrease of the soft tissue mass, but the lytic lesion of the sternum remained unchanged. The designation of response to treatment was difficult on CT. (d) FDG-PET showed no residual uptake in the previously involved sternum, but diffuse uptake of the whole bone marrow consistent with marrow regeneration due to growth factor treatment.
Early response to first-line treatment
The clinical parameters incorporated in the International Prognostic Index (IPI) grossly reflect the biological heterogeneity of lymphoma. In this respect, the duration of a complete remission might be significantly more influenced by the chemosensitivity than by the initial IPI factors [7]. Consequently, an early evaluation during treatment leading to an alternative treatment might improve outcome. Several studies have established that interim FDG-PET scans after 1–3 cycles of chemotherapy provide valuable information regarding early assessment of response and survival. Conventional chemotherapy can induce a rapid decrease of FDG uptake as soon as 7 days after treatment [16]. Spaepen et al. have shown the important prognostic value of mid-treatment FDG-PET in monitoring 70 cases of aggressive NHL [17]. Thirty-three patients showed persistence of abnormal FDG uptake and none of them achieved a durable complete remission, whereas 37 showed a negative scan. Out of the 37 patients, 31 remained in compete remission. More recently, we have confirmed the early (after two cycles) prognostic impact of FDG-PET in terms of response and survival [16]. At mid-induction, ‘early PET’ was considered negative in 54 patients and positive in 36. The outcome differed significantly between PET-negative and PET-positive groups; the predictive value of ‘early PET’ was observed in both the lower-risk and higher-risk groups, indicating prognostic independence from IPI. Therefore, FDG-PET should guide first-line strategies in lymphoma. The role of PET scanning for post-therapy surveillance without clinical or biochemical or CT evidence of disease (complete remission status) remains controversial primarily because of the potential for a disproportionate fraction of false-positive findings potentially resulting in increased cost without proven benefit from early PET detection of disease compared with standard conventional methods [18]. Large prospective studies are therefore needed to determine whether routine surveillance by PET results in meaningful changes in patient management [14].
MRI
T2 signal
The signal intensity of lymphoma at MR imaging changes during the course of the disease [19]. Active untreated tumour tissue contains an excess of free water which increases the signal intensity on T2 WI. With successful treatment, cellular elements and the water content of the tumour are reduced while the collagen and fibrotic stroma of the original tumour account for the main component of the signal [20]. These factors reduce the signal intensity of the residual mass on T2 WI and have been used for predicting relapse in a residual mass. However, the sensitivity of MR imaging in the prediction of relapse in a residual mass ranges from 45% to 90%, with a specificity ranging from 80% to 90% [21, 22]. Low sensitivity is mainly due to necrosis, immature fibrotic tissue, oedema and inflammation that can simulate the high T2 SI of a viable tumour.
Gadolinium injection
Gadolinum (Gd) enhancement of lymphoma of the mediastinum changes during the course of the disease. The mean Gd enhancement of residual masses after treatment is substantially weaker than that observed before treatment in patients in complete remission. Enhancement of these inactive residual masses decreases markedly to the same level as that of muscle [23]. This may be explained by a higher degree of vascularization and a larger extracellular compartment in the active cellular tumour compared with dense immature fibrotic tissue. Due to different enhancement of lymphomatous masses at diagnosis, MR evaluation of residual masses requires a pre-treatment baseline MR study for comparison. Further studies with more recent MR techniques of perfusion analysis are required for comparison with FDG-PET. MR imaging suffers from limited field of view analysis compared to MDCT and PET. Furthermore, various impairments such as motion artefacts alter the overall image analysis especially in the mediastinum.
Potential of MRI
Preliminary studies have suggested a potential role of diffusion MR imaging in oncologic patients by allowing the detection of water motion over small distances [24]. The development of body MR using multi-channel phased array surface coils combined with parallel imaging techniques could enable whole body MR diffusion imaging in cancer patients. No study has yet been published assessing the impact of such techniques in staging and monitoring lymphoma.
New contrast agents taken up by the reticulo-endothelial system are now available, such as superparamagnetic iron oxides (USPIO). USPIO are taken up by normal and hypercellular bone marrow, but not by neoplastic lesions, thereby providing significantly different enhancement patterns on T2-weighted MR images [25]. USPIO could therefore help in differentiating normal from lymphomatous bone marrow.
MRI in bone marrow involvement
In patients with lymphoma, MR identification of focal lesions within the bone marrow is important for patient staging according to the Ann Arbor classification. BMB remains the gold standard in this setting, but its sensitivity is sub-optimal as the biopsy site may not reflect the entire bone marrow compartment. Bilateral iliac crest BMB usually increases the sensitivity of unilateral BMB. Early during bone marrow infiltration, tumour cells do not displace bone marrow fat cells; the amount of fat cells remains normal. Subsequently, replacement of normal marrow by tumour cells leads to a reduction in T1 SI and an increase in T2 SI. Focal lesions are easily detected when using appropriate MR techniques such as fat suppressed T2 WI before treatment. Several studies have shown that NHL induces angiogenesis [26]. Dynamic contrast-enhanced MR images can demonstrate increased bone marrow enhancement in patients with lymphoproliferative diseases and marrow involvement (Fig. 4) [27, 28]. Contrast enhancement decreases after treatment in good responders.
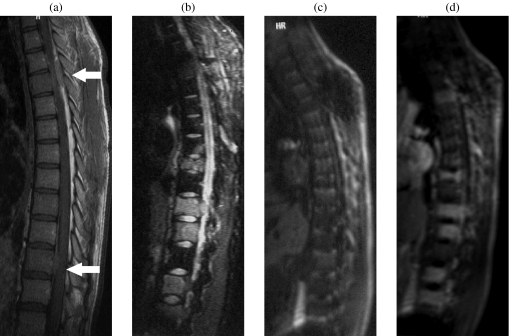
Figure 4.
A 28-year-old patient with NHL with spine and epidural involvement. (a) T1 WI showed two epidural masses (arrows). The bone marrow signal is consistent with the young age of the patient or with diffuse involvement. (b) Fat suppressed T2 WI demonstrated multiple focal bone marrow lesions. (c) T1 GE WI before injection and (d) 35 s after Gd-chelates injection showed early enhancement of focal lesions without enhancement of the remaining marrow. Bone marrow biopsy in the iliac crest was normal.
Conclusion
Both FDG-PET and MRI can provide whole body imaging. Both also share the advantage of combining functional and anatomical information. Hybrid FDG-PET and MDCT is the best technique for monitoring response to treatment, especially early response to treatment. Early assessment of response to treatment has the potential to tailor therapy. MR imaging is especially useful in assessing bone marrow and central nervous system involvement.
References
- 1.Haioun C, Lepage E, Gisselbrecht C, et al. High-dose therapy followed by stem cell transplantation in partial response after first-line induction therapy for aggressive non-Hodgkin’s lymphoma. Ann Oncol. 1998;9((Suppl 1)):S5–8. doi: 10.1093/annonc/9.suppl_1.s5. [DOI] [PubMed] [Google Scholar]
- 2.Dugdale PE, Miles KA, Bunce I, Kelley BB, Leggett DA. CT measurement of perfusion and permeability within lymphoma masses and its ability to assess grade, activity, and chemotherapeutic response. J Comput Assist Tomogr. 1999;23:540–7. doi: 10.1097/00004728-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Shalom R, Yefremov N, Haim N, et al. Camera-based FDG PET and 67Ga SPECT in evaluation of lymphoma: comparative study. Radiology. 2003;227:353–60. doi: 10.1148/radiol.2272020195. [DOI] [PubMed] [Google Scholar]
- 4.Janicek M, Kaplan W, Neuberg D, Canellos GP, Shulman LN, Shipp MA. Early restaging gallium scans predict outcome in poor-prognosis patients with aggressive non-Hodgkin’s lymphoma treated with high-dose CHOP chemotherapy. J Clin Oncol. 1997;15:1631–7. doi: 10.1200/JCO.1997.15.4.1631. [DOI] [PubMed] [Google Scholar]
- 5.Kazama T, Faria SC, Varavithya V, Phongkitkarun S, Ito H, Macapinlac HA. FDG PET in the evaluation of treatment for lymphoma: clinical usefulness and pitfalls. Radiographics. 2005;25:191–207. doi: 10.1148/rg.251045045. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor V, McCook BM, Torok FS. An introduction to PET-CT imaging. Radiographics. 2004;24:523–43. doi: 10.1148/rg.242025724. [DOI] [PubMed] [Google Scholar]
- 7.Haioun C, Itti E, Rahmouni A, Meignan M, Reyes F. PET scan in the therapeutic strategy. Hematol J. 2004;5((Suppl 3)):S149–53. doi: 10.1038/sj.thj.6200442. [DOI] [PubMed] [Google Scholar]
- 8.Moog F, Bangerter M, Diederichs CG, et al. Lymphoma: role of whole-body 2-deoxy-2-[F-18]fluoro-D-glucose (FDG) PET in nodal staging. Radiology. 1997;203:795–800. doi: 10.1148/radiology.203.3.9169707. [DOI] [PubMed] [Google Scholar]
- 9.Schoder H, Meta J, Yap C, et al. Effect of whole-body (18)F-FDG PET imaging on clinical staging and management of patients with malignant lymphoma. J Nucl Med. 2001;42:1139–43. [PubMed] [Google Scholar]
- 10.Castellucci P, Nanni C, Farsad M, et al. Potential pitfalls of 18F-FDG PET in a large series of patients treated for malignant lymphoma: prevalence and scan interpretation. Nucl Med Commun. 2005;26:689–94. doi: 10.1097/01.mnm.0000171781.11027.bb. [DOI] [PubMed] [Google Scholar]
- 11. Castellucci P, Zinzani P, Pourdehnad M, (18)F-FDG PET in malignant lymphoma: significance of positive findings. Eur J Nucl Med Mol Imaging 2005 (Epub ahead of print)
- 12.Jerusalem G, Beguin Y, Fassotte MF, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin’s disease and non-Hodgkin’s lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood. 1999;94:429–33. [PubMed] [Google Scholar]
- 13.Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin’s lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001;19:414–19. doi: 10.1200/JCO.2001.19.2.414. [DOI] [PubMed] [Google Scholar]
- 14.Juweid ME, Cheson BD. Role of positron emission tomography in lymphoma. J Clin Oncol. 2005;23:4577–80. doi: 10.1200/JCO.2005.01.904. [DOI] [PubMed] [Google Scholar]
- 15.Juweid ME, Wiseman GA, Vose JM, et al. Response assessment of aggressive non-Hodgkin’s lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005;23:4652–61. doi: 10.1200/JCO.2005.01.891. [DOI] [PubMed] [Google Scholar]
- 16. Haioun C, Itti E, Rahmouni A, [ 18F]Fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood 2005 (Epub ahead of print)
- 17.Spaepen K, Stroobants S, Dupont P, et al. Early restaging positron emission tomography with (18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:1356–63. doi: 10.1093/annonc/mdf256. [DOI] [PubMed] [Google Scholar]
- 18.Jerusalem G, Beguin Y, Fassotte MF, et al. Early detection of relapse by whole-body positron emission tomography in the follow-up of patients with Hodgkin’s disease. Ann Oncol. 2003;14:123–30. doi: 10.1093/annonc/mdg011. [DOI] [PubMed] [Google Scholar]
- 19.Rahmouni A, Tempany C, Jones R, Mann R, Yang A, Zerhouni E. Lymphoma: monitoring tumor size and signal intensity with MR imaging. Radiology. 1993;188:445–51. doi: 10.1148/radiology.188.2.8327695. [DOI] [PubMed] [Google Scholar]
- 20.Nyman RS, Rehn SM, Glimelius BL, Hagberg HE, Hemmingsson AL, Sundstrom CJ. Residual mediastinal masses in Hodgkin disease: prediction of size with MR imaging. Radiology. 1989;170:435–40. doi: 10.1148/radiology.170.2.2911665. [DOI] [PubMed] [Google Scholar]
- 21.Gasparini MD, Balzarini L, Castellani MR, et al. Current role of gallium scan and magnetic resonance imaging in the management of mediastinal Hodgkin lymphoma. Cancer. 1993;72:577–82. doi: 10.1002/1097-0142(19930715)72:2<577::aid-cncr2820720240>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Devizzi L, Maffioli L, Bonfante V, et al. Comparison of gallium scan, computed tomography, and magnetic resonance in patients with mediastinal Hodgkin’s disease. Ann Oncol. 1997;8((Suppl 1)):53–6. [PubMed] [Google Scholar]
- 23.Forsgren G, Nyman R, Glimelius B, Hagberg H, Rehn S, Hemmingsson A. Gd-DTPA-enhanced MR imaging in mediastinal Hodgkin’s disease. Acta Radiol. 1994;35:564–9. [PubMed] [Google Scholar]
- 24.Sato C, Naganawa S, Nakamura T, et al. Differentiation of noncancerous tissue and cancer lesions by apparent diffusion coefficient values in transition and peripheral zones of the prostate. J Magn Reson Imaging. 2005;21:258–62. doi: 10.1002/jmri.20251. [DOI] [PubMed] [Google Scholar]
- 25.Daldrup-Link HE, Rummeny EJ, Ihssen B, Kienast J, Link TM. Iron-oxide-enhanced MR imaging of bone marrow in patients with non-Hodgkin’s lymphoma: differentiation between tumor infiltration and hypercellular bone marrow. Eur Radiol. 2002;12:1557–66. doi: 10.1007/s00330-001-1270-5. [DOI] [PubMed] [Google Scholar]
- 26.Ribatti D, Vacca A, Nico B, Fanelli M, Roncali L, Dammacco F. Angiogenesis spectrum in the stroma of B-cell non-Hodgkin’s lymphomas. An immunohistochemical and ultrastructural study. Eur J Haematol. 1996;56:45–53. doi: 10.1111/j.1600-0609.1996.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 27.Montazel JL, Divine M, Lepage E, Kobeiter H, Breil S, Rahmouni A. Normal spinal bone marrow in adults: dynamic gadolinium-enhanced MR imaging. Radiology. 2003;229:703–9. doi: 10.1148/radiol.2293020747. [DOI] [PubMed] [Google Scholar]
- 28.Rahmouni A, Montazel JL, Divine M, et al. Bone marrow with diffuse tumor infiltration in patients with lymphoproliferative diseases: dynamic gadolinium-enhanced MR imaging. Radiology. 2003;229:710–17. doi: 10.1148/radiol.2293020748. [DOI] [PubMed] [Google Scholar]