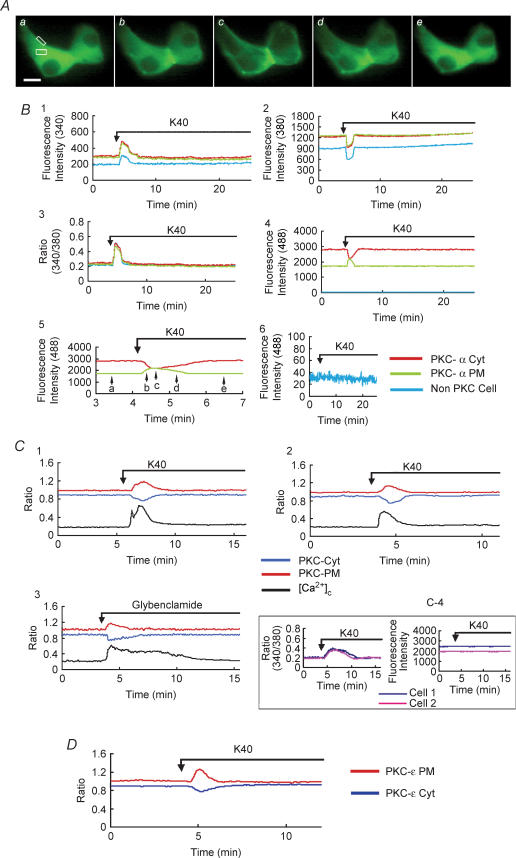
Figure 1. Effect of high concentration of K+ and glybenclamide on translocation of PKC.
A, images of PKC-α–EGFP translocation induced by 40 mm KCl at high time resolution (sample interval, 5 s). Panels a–e were taken at the times indicated by the arrows in B5. The scale bar represents 10 μm. The image is representative of 296 images from 18 independent experiments. B, the entire time course of simultaneous measurements of [Ca2+]c, PKC-α Cyt and PKC-α PM in islet cell transfected with PKC-α–EGFP and loaded with fura-2. B1, the fluorescence was excited at 340 nm. The fluorescence intensity in the regions of interest (white boxes in A) in a PKC-α–EGFP-expressing cell and a non-PKC-α–EGFP-expressing cell within the same monitor field (cell image not shown) was recorded. B2, the fluorescence was excited at 380 nm. The fluorescence intensity in the regions of interest was recorded. B3, the ratio of fluorescence 340/380 nm was recorded as the ratio of [Ca2+]c. B4, the fluorescence was excited at 488 nm. The fluorescence intensity in the regions of interest was recorded. The fluorescence in a non-PKC-α–EGFP-expressing cell was seen as almost a straight line on the X-axis indicating that the influence of fura-2 fluorescence on GFP fluorescence was negligible. B5, fluorescence changes shown in B4 with higher time resolution. B6, the fluorescence intensity was recorded in a non-PKC-α–EGFP-expressing cell loaded with fura-2 with excitation at 488 nm. No significant change was recorded during stimulation. The translocation of PKC-α–EGFP in PKC-α–EGFP-expressing cell not loaded with fura-2 was also monitored (data not shown). The time course of translocation is similar to the data shown in B5. Therefore, the crossover between fura-2 and EGFP images was negligible. C1, islet cells were transfected with PKC-θ–EGFP and loaded with fura-2. The cell was stimulated by 40 mm KCl and changes in the fluorescence of PKC-θ in cytosol (PKC-Cyt), PKC-θ in the plasma membrane (PKC-PM) and [Ca2+]c were monitored. The value of each EGFP fluorescence intensity (F) was normalized to the initial value (F0), so that the relative fluorescence change was referred to as the ratio F /F0. In order to distinguish the fluorescence ratio of PKC in the cytosol (PKC-Cyt) from the PKC in the plasma membrane (PKC-PM), the ratio of PKC-Cyt was modified as F /F0− 0.1 in the figure. The traces are representative of 73 traces from 9 independent experiments. C2, islet cells were transfected with C1-EGFP and loaded with fura-2. The cell was stimulated by 40 mm KCl and changes in the fluorescence from C1 in the cytosol (PKC-Cyt), plasma membrane (PKC-PM) and [Ca2+]c were monitored. Data are shown in the same way as described in C1. The traces are representative of 24 traces from 3 independent experiments. C3, islet cells were transfected with PKC-α–EGFP and loaded with fura-2. The cell was stimulated by 10 μm glybenclamide and changes in the fluorescence from PKC-α in the cytosol (PKC-Cyt), in the plasma membrane (PKC-PM) and [Ca2+]c were monitored. The traces are representative of 36 traces from 5 independent experiments. C4, islet cells were transfected with EGFP-N2 and loaded with fura-2. The cells were stimulated by 40 mm KCl and changes in the fluorescence from EGFP (right) in cytosol and [Ca2+]c (left) of two cells were monitored. [Ca2+]c was changed significantly after the stimulation of 40 mm KCl, while there were no apparent changes in the fluorescence from EGFP indicating that there is no apparent crossover between the fluorescence of fura-2 and EGFP. The traces are representative of 20 traces from 3 independent experiments. D, islet cells were transfected with PKC-ɛ–GFP. The cell was stimulated by 40 mm KCl.