Abstract
We have defined Ca2+ channel subtypes expressed in rabbit carotid body (CB) chemoreceptor cells and their participation in the stimulus-evoked catecholamine (CA) release. Ca2+ currents (ICa) activated at –30 mV, peaked at +10 mV and were fully blocked by 200 μm Cd2+. L-type channels (sensitive to 2 μm nisoldipine) activated at –30 mV and carried 21 ± 2% of total ICa. Non-L-type channels activated at potentials positive to –10 mV and carried: N channels (sensitive to 1 μm ω-conotoxin-GVIA) 16 ± 1% of total ICa, P/Q channels (sensitive to 3 μm ω-conotoxin-MVIIC after nisoldipine plus GVIA) 23 ± 3% of total ICa and R channels (resistant to all blockers combined) 40 ± 3% of total ICa. CA release induced by hypoxia, hypercapnic acidosis, dinitrophenol (DNP) and high K+o in the intact CB was inhibited by 79–98% by 200 μm Cd2+. Hypoxia, hypercapnic acidosis and DNP, depolarized chemoreceptor cells and eventually generated repetitive action potential discharge. Nisoldipine plus MVIIC nearly abolished the release of CAs induced by hypoxia and hypercapnic acidosis and reduced by 74% that induced by DNP. All these secretory responses were insensitive to GVIA. 30 and 100 mm K+o brought resting membrane potential (Em) of chemoreceptor cells (–48.1 ± 1.2 mV) to –22.5 and +7.2 mV, respectively. Thirty millimolar K+o-evoked release was abolished by nisoldipine but that induced by 100 mm K+o was mediated by activation of L, N, and P/Q channels. Data show that tested stimuli depolarize rabbit CB chemoreceptor cells and elicit CA release through Ca2+ entry via voltage-activated channels. Only L and P/Q channels are tightly coupled to the secretion of CA.
The carotid body (CB) is a secondary sensory organ activated by low PO2, acidosis and hypercapnia. The sensing structures of the CB are chemoreceptor or type I cells, which are innervated by fibres of the carotid sinus nerve (CSN), a branch of the IXth cranial nerve. Physiological stimuli augment the release of neurotransmitters, and thereby the frequency of action potential in the CSN, whose central projections end up in the nucleus tractus solitarius, originating a reflex hyperventilation aimed to normalize arterial blood gases and pH. Metabolic poisons and several neuromodulators also activate the organ (Gonzalez et al. 1994). Among the neurotransmitters so far identified in chemoreceptor cells, catecholamine (CA; mostly dopamine, DA) have been the more extensively studied. DA is released in proportion to the intensity of the stimulus and to the action potential frequency recorded in the CSN (Fidone et al. 1982; Gonzalez et al. 1994; Montoro et al. 1996).
After the pioneer study in isolated cultured chemoreceptor cells demonstrating the presence of Na+, K+ and Ca2+ voltage-activated currents (Lopez-Barneo et al. 1988), several works have been directed to characterize the calcium currents (ICa) expressed in these cells. In spite of species differences, all studies agree on the presence of L- and non-L-type Ca2+ channels in CB chemoreceptor cells (e.g. Fieber & McCleskey, 1993; Silva & Lewis, 1995; Peers et al. 1996; Overholt & Prabhakar, 1997; Rocher et al. 1999). The presence of L-type voltage-activated Ca2+ channels in chemoreceptor cells was first demonstrated in CA release experiments performed in an in vitro preparation of the intact CB. In these studies it was shown that organic agonists and antagonists of L-type channels modified in the predicted direction the high K+o and the low PO2-evoked release of CA (Obeso et al. 1992). These results were later supported by studies in isolated cultured cells in which it was shown that hypoxia and high K+o augments Ca2+i, these increases being reduced by dihydropyridine treatment (Buckler & Vaughan-Jones, 1994a; Ureña et al. 1994; Jiang & Eyzaguirre, 2004). While the role of L-type Ca2+ channels in the CA release from chemoreceptor cells has been unambiguously established, the significance of non-L-type channels for the release of neurotransmitters has not been investigated. Existing data suggest that their role modulating the evoked release of CA may depend on the stimulus modality and intensity. Thus, while the release response to high K+o was almost abolished by dihydropyridines (Obeso et al. 1992; Hatton & Peers, 1997), that induced by low PO2 presented a variable sensitivity to these drugs, with a response progressively less sensitive to dihydropyridines as the intensity of hypoxia increased (Obeso et al. 1992). Contrary to the well established participation of voltage-activated calcium current in the release of CA induced by high K+o and hypoxia, current understanding of the calcium entry pathways activated during acid and dinitrophenol (DNP) stimulation varies with the studied species: voltage-dependent calcium channels in rat CB and Na+/Ca2+ exchangers (NCXs) working in reverse mode in the rabbit CB (Rocher et al. 1991; Buckler & Vaughan-Jones, 1994b).
Using inorganic and organic blockers of calcium channels, we have investigated (i) the types of calcium channels expressed in chemoreceptor cells of the rabbit CB and (ii) their involvement in the secretion of CA induced by moderate and intense hypoxia, hypercapnic acidosis, DNP and 30 and 100 mm K+o. In addition, we have characterized the effect of these stimuli on the membrane potential of chemoreceptor cells. Our results demonstrate the existence of L, N, P/Q and R channels in these cells; only L and P/Q channels were tightly coupled to the stimulus-evoked release of CA. In all cases, the secretion of CA was almost abolished by inorganic and organic blockers of Ca2+ channels, showing that this secretion is secondary to the entry of Ca2+ to the cytosol through voltage-dependent Ca2+ channels. This hypothesis is supported by electrophysiological recordings showing membrane depolarization of chemoreceptor cells during the application of all tested stimuli. Preliminary results have been published by Rocher et al. (2003).
Methods
Cell preparation and electrophysiological recordings
Chemoreceptor cells were obtained from the CB of adult New Zealand White rabbits anaesthetized with sodium pentobarbital (40 mg kg−1, Sigma) administered through the lateral vein of the ear. Animals were killed with an intracardiac overdose of pentobarbital (100–200 mg). Experimental procedures were approved by the Institutional Animal Care and Use Committee of the Universities of Valladolid and Miguel Hernández and have been described in detail elsewhere (Rocher et al. 1991).
The CBs (usually two) were incubated for 30 min in nominally Ca2+ and Mg2+-free Tyrode solution (pH 7.2) containing collagenase (2.5 mg ml−1, type IV, Sigma) and bovine serum albumin (6 mg ml−1, Fraction V, Sigma). After centrifugation (800 g, 5 min), the pellet was placed in new solution containing collagenase (1 mg ml−1), trypsin (1 mg ml−1, type II, Sigma) and bovine serum albumin (6 mg ml−1) for an additional 15-min period. During both incubation periods the tissues were subjected to mechanical disruption every 10 min by repeated aspiration through a fire-polished Pasteur pipette. After centrifugation (800 g, 8 min), the cells were washed in an enzyme-free Tyrode solution and thereafter placed in 100 μl of culture medium (DMEM:F-12, Sigma), supplemented with 5% fetal bovine serum, 2 mml-glutamine, 100 μg ml−1 streptomycin, and 40 μg ml−1 gentamicin. Dispersed cells were plated as 10–20 μl drops on small poly l-lysine-coated coverslips kept in 3.5 cm diameter Petri dishes and maintained in a humidified incubator (37°C; 5% CO2 in air). Once the cells attached, 2 ml of culture medium was added to maintain the cells until use (3–36 h later). Coverslips were transferred to a small recording chamber (0.15 ml volume; Warner Instrument Corporation, Hamden, CT, USA) on the stage of an inverted microscope (Nikon Diaphot-TMD) and superfused by gravity (1.5–3 ml min−1).
Recordings of calcium currents in chemoreceptor cells: voltage-clamp experiment
These experiments were performed at room temperature (20–23°C) using the patch-clamp technique in either the whole-cell or the perforated-patch configuration. Gigaseals were formed in Hepes-buffered solution containing (mm): NaCl 130; KCl 5; CaCl2 10; Hepes 10, glucose 5, and tetrodotoxin 5 × 10−7m (TTX, Alomone Laboratories), pH adjusted to 7.42 with NaOH. Patch pipettes of borosilicate glass (1.5 mm o.d.; Clark Electromedical Instruments) were filled with a solution containing (mm): CsCl 130; MgCl2 2; Hepes 10; ethylene glycol-bis(β-aminoethylether)-n,n,n′,n′-tetraacetic acid (EGTA) 10. ATP (4 mm) and GTP (2 mm) were also present in this solution in the whole-cell recordings, while nystatin (100–150 μg ml−1) was added to the solution used in the perforated-patch recordings (Albillos et al. 2000). The final pH of intracellular solutions was adjusted to pH 7.2 with CsOH. The resistance of pipettes filled with internal solution was 2.0–3.5 MΩ. In the whole-cell recordings, the holding potential was set at −80 mV and during the experimental trial it was stepped in 10 mV increments (from −70 to +70 mV; 20 ms duration). To evaluate the effect of the Ca2+ channel agonist/antagonist on ICa, two consecutive current–voltage (I–V) relationships were performed in control (drug-free) solution, 1.5 min after the addition of the drug and 2–6 min after returning to control solution. The current amplitude in every cell was normalized as I/Imax, where Imax was the maximal current obtained in control solution (usually during pulse depolarization to +10 or +20 mV). Data are expressed as mean ± s.e.m. of the normalized currents. In the perforated-patch recordings, the membrane potential was clamped at –80 mV, and depolarizing pulses to +10 mV (60–100 ms duration) were applied every 10 s. All calcium currents were leak subtracted.
Recordings of membrane potential in chemoreceptor cells: current-clamp experiments
All these experiments were performed using the perforated-patch configuration and at 33–35°C. Bath solutions were bicarbonate buffered and identical to those used in the [3H]CA release experiments (see below). Pipette solution was (mm): KCl 35; potassium gluconate 95; MgCl2 3; EGTA 5; Hepes 10; pH was adjusted to 7.2 by addition of NaOH (final sodium concentration 14 mm). Nystatin was added at a final concentration of 100–150 μg ml−1. To test the effect of hypoxia on membrane potential, cells were superfused with a solution pre-equilibrated in the reservoir with 95% N2–5% CO2. Measurements of PO2 in the recording chamber were performed with an O2 microelectrode (Diamond General Corp.) and they rendered values of 20–30 mmHg.
Current and voltage signals were recorded with an EPC-7 amplifier (List Medical, Darmstadt, Germany) or an RK 300 amplifier (BioLogic, Claix, France). Pulse generation, data acquisition and analysis were made through an A-D converter (CED 1401, Cambridge, UK) or through a Digidata 1322 A (Axon Instruments, California, USA) commanded by the software package ‘Strathclyde Electrophysiology Software’ (kindly provided by J. Dempster, Strathclyde University) or pCLAMP software (Axon Instruments). Current and voltage recordings were filtered at 2 KHz and sampled at 10–16 KHz.
[3H]CA release experiments
Right and left CBs of 4–6 rabbits were incubated separately for 2 h (37°C) in a Tyrode solution equilibrated with 100% O2 (mm: NaCl 140; KCl 5; CaCl2 2; MgCl2 1; Hepes 10; glucose 5; pH adjusted to 7.42 with NaOH) containing 30 × 10−6 m[3H]tyrosine (30 Ci mmol−1; Amersham), 10−4 md,l-6-methyl-5,6,7,8-tetrahydropterine (Sigma) and 10−3m ascorbic acid to label CA stores (Rocher et al. 1991). Thereafter, individual CBs were incubated at 37°C in a bicarbonate-buffered solution (mm: NaCl 116; NaHCO3 24; KCl 5; CaCl2 2; MgCl2 1; Hepes 10; glucose 5; pH 7.42) equilibrated with 20% O2–5% CO2–75% N2 that was renewed every 30 min and discarded. After 2 h, the solutions were renewed every 10 min and collected for later analysis of their [3H]CA content. The general protocol in these experiments is presented in Fig. 1B. Every CB was subjected to two consecutive identical stimuli (S1 and S2, respectively). The stimuli consisted of the incubation of the organ in low PO2-equilibrated solutions (7% O2–5% CO2–88% N2 or 2% O2–5% CO2–93 N2), K+-enriched solutions (30 or 100 mm), acidic hypercapnic solution (20% O2–20% CO2–60% N2; pH 6.6) or control (normoxic) solution (equilibrated with 20% O2–5% CO2–75% N2) containing DNP (50, 100 or 200 μm) for a period of 10 (or 5) min. In the high-K+ solutions, equimolar amounts of Na+ were removed to maintain the osmolarity. Composition of acidic hypercapnic solution was (mm): NaCl 126; NaHCO3 14; KCl 5; CaCl2 2; MgCl2 1.1; Hepes 10; glucose 5; pH 6.6 when equilibrated with 20% O2–20% CO2–60% N2. The stimulus-evoked release of [3H]CA in S1 and S2 was calculated as counts per minute (c.p.m) above basal release (c.p.m. above the dashed line in the Fig. 1B) and expressed as a percentage of the [3H]CA present in the organ immediately before the application of the stimulus. Control and experimental CBs were stimulated as described but in the latter group, the calcium antagonist tested was present in the incubating solutions 10 min before and during the application of S2 (in some cases it was also present in the first post-stimulus period). In every 10 min incubation period, the solution was continuously bubbled through a fine needle to assure maintenance of the PO2/PCO2 at the desired level. At the end of the experiments, the CBs were homogenized in 400 μl 0.4 m PCA and centrifuged for 5 min in a microfuge (Beckman). The [3H]CA present in the CB supernatant and in the collected incubation solutions was adsorbed onto 100 mg acid-washed alumina (Bio-Rad) at pH 8.6, batch eluted with 1 ml 1 m HCl and counted in a scintillation spectrometer. The effect of a drug on the stimulus-induced release of [3H]CA was evaluated comparing the S2/S1 ratio (evoked release by S2/evoked release by S1) obtained in control versus drug-treated CB. Statistical significance of the observed differences was assessed using a two-tailed Student's t test for unpaired data; the significance level was established at P < 0.05. Results are expressed as mean ± s.e.m. and in some cases, referred to as a percentage of the control release (100%) obtained in control (untreated) CBs. The experiments were designed in such a way that experimental CBs had their contralateral CB as controls. There were no statistically significant differences in the magnitude of S1-evoked release between control and experimental CB in any experimental condition.
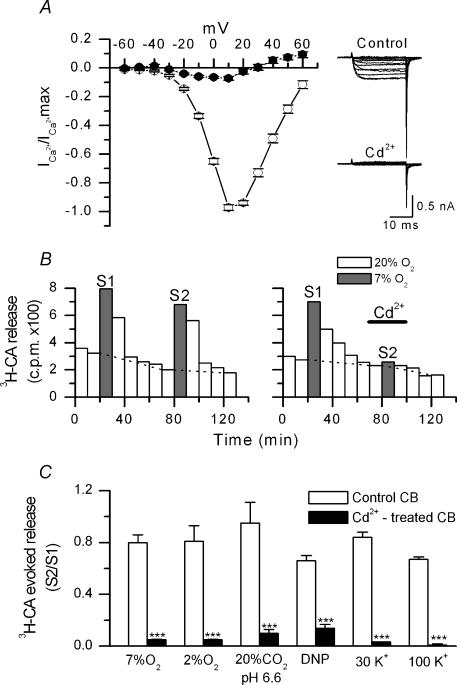
Figure 1. Effect of cadmium on calcium currents and stimulus-induced secretion of [3H]CA.
A, normalized I–V relationships obtained in isolated chemoreceptor cells before (○) and during (•) superfusion with 200 μm CdCl2 (n = 4). From a holding potential of –80 mV, currents were elicited by 20 ms depolarizing pulses in 10 mV intervals. On the right, single currents obtained in a representative cell in both conditions. B. general protocol used in the experiments of [3H]CA release. The figure shows the actual release of [3H]CA (c.p.m.) from a control CB (left panel) incubated in normoxia, a 20% O2–5% CO2–75% N2-equilibrated solution (PO2∼150 mmHg; open bars), or hypoxia, a 7% O2–5% CO2–88% N2-equilibrated solution (PO2∼46 mmHg; grey bars). Hypoxia was applied twice (S1 and S2). Evoked release in every application of hypoxic stimuli corresponds to the sum of c.p.m. above dashed lines. In the experimental CB (right panel), the protocol was identical except for the presence of 200 μm CdCl2 during the time indicated by the horizontal line. C, effect of CdCl2 (200 μm) on the evoked release induced by mild (7% O2-equilibrated solution) and intense (2% O2-equilibrated solution) hypoxia, hypercapnic acidosis (20% CO2-equilibrated solution; pH 6.6), dinitrophenol (DNP; 100 μm), and 30 and 100 mm extracellular K+o. Experimental protocol as in B. For every type of stimulation, the figure shows average ratios of the evoked release in S2 to the evoked release in S1 (S2/S1), in control and cadmium-treated CB (n = 5–12 in every experimental condition; ***P < 0.001).
Drugs
ω-Conotoxin GVIA (GVIA) and tetrodotoxin (TTX) were purchased from Alomone (Alomone Laboratories Jerusalem, Israel), ω-conotoxin MVIIC from Bachem (Bachem AG, Switzerland) and (S)-(–)-Bay K8644 from Sigma (Sigma-Aldrich, Madrid). Nisoldipine was a gift from Professor A. García (Department of Pharmacology, Universidad Autónoma de Madrid).
Results
Cadmium blocks voltage-dependent calcium channels and the release of [3H]CA induced by hypoxia, hypercapnic acidosis, DNP and high K+o
Voltage-activated ICa of isolated rabbit chemoreceptor cells was first studied using the whole-cell configuration of patch-clamp technique. In cells voltage clamped at −80 mV and in the presence of 10 mm external Ca2+, the current activated at ∼−30 mV, peaked between +10 and +20 mV and showed an apparent reversal potential around +60 mV. Mean maximum current obtained in 33 cells was 604 ± 22 pA (range 170–1120 pA). As shown in Fig. 1A, the ICa of chemoreceptor cells was completely blocked by cadmium at low concentration (CdCl2 200 μm).
The effect of cadmium on the release of [3H]CA evoked by different types of stimulation was studied following a protocol similar to that shown in Fig. 1B for mild (7% O2) hypoxia (see Methods). Some stimuli were more effective than others in producing release of [3H]CA from the CB. Thus, the evoked release during the first (S1) stimulation cycle (expressed as a percentage of the total 3H-CA present in the CB before stimulation) was: 1.91 ± 0.16% for 7% O2; 7.04 ± 0.35% for 2% O2; 0.64 ± 0.08% for hypercapnic acidosis (20% CO2; pH 6,6); 9.00 ± 0.73% for 100 μm DNP; 2.91 ± 0.11% for 30 mm K+o, and 28.46 ± 0.80% for 100 mm K+o (stimulus duration was 10 min except for 100 mm K+ that was 5 min). These responses correspond to mean release responses obtained in 35–70 trials of the different stimuli made in the entire study. Despite these differences in the magnitude of the responses, Cd2+ reduced drastically all the stimulus-induced secretory responses (Fig. 1C). The degree of inhibition oscillated between 98% (for 100 mm K+) and 79% (for DNP). These data indicate that entry of calcium through voltage-dependent channels is necessary to promote the secretory response to hypoxia, acidosis, DNP and high K+o.
Types of voltage-activated calcium channels expressed in chemoreceptor cells
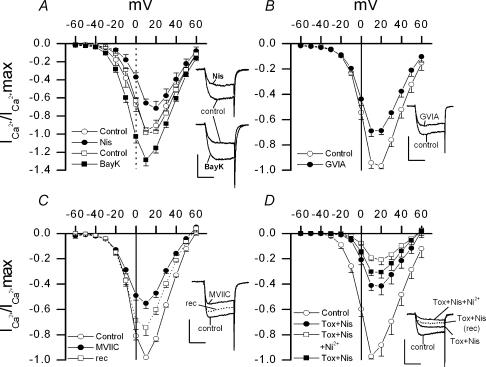
We used pharmacological tools to identify the calcium channels subtypes expressed in chemoreceptor cells. Calcium currents were recorded under whole-cell configuration in the absence and in the presence of specific agonists or antagonists of different calcium channels types. Figure 2A shows the presence in these cells of L-type channels. Nisoldipine (2 μm), a specific antagonist of L-type Ca2+ channels, reduced by 65 ± 6% (n = 6; range 30%-74%) the current obtained at −20 mV, this effect being less intense at more depolarized potentials (i.e. 31 ± 5% inhibition at +10 mV). Bay K8644 (1.5 μm), a specific agonist of these channels, augmented ICa in a strongly voltage-dependent manner: at −20 mV Bay K8644 increased the current by 155 ± 26% (n = 6; range 61–258%) while at +10 mV the currents augmented by 30 ± 6% (range 11%-54%). As reported in other cell types possessing L-type currents, Bay K8644 dramatically slowed tail current decay. The activation threshold of the calcium current sensitive to dihydropyridines was ∼−30 mV.
Figure 2. Effect of nisoldipine (Nis), Bay K8644 (BayK), ω-conotoxin GVIA (GVIA), ω-conotoxin MVIIC (MVIIC) and nickel (Ni2+) on the calcium currents of isolated chemoreceptor cells.
Calcium currents were recorded using the whole-cell configuration of the patch-clamp technique. A, B and C show averages of normalized I–V relationships obtained in chemoreceptor cells before and during superfusion with 2 μm Nis and 1.5 μm BayK (A); 1 μm GVIA (B); and 3 μm MVIIC (C). Current recovery after washout of MVIIC is also show in C. In D, I–V curves were obtained in control conditions, in the presence of a cocktail containing nisoldipine (Nis; 2 μm) and toxins (1 μm GVIA plus 3 μm MVIIC; tox), and in the presence of that drug mixture plus NiCl2 (100 μm). Current recovery after washout of Ni2+ is also shown. Voltage protocols as in Fig. 1A. Sample records of currents obtained in individual cells at voltage pulses to 0 mV (A) or +10 mV (B, C and D) are shown in the insets (calibration bars 0.5 nA and 10 ms). n = 5–7 cells in every case.
At concentrations of 1 μm, the ω-conotoxin GVIA (GVIA) blocks neuronal N-type Ca2+ channels in an irreversible and specific manner (Hillyard et al. 1992; Mintz et al. 1992; Olivera et al. 1994; McDonough et al. 1996, 2002; Wakamori et al. 1998). In isolated chemoreceptor cells, this concentration of GVIA (1 μm) reduced by 27 ± 5% the peak current obtained at +10 mV (n = 5; range of inhibition 15–35%; see Fig. 2B). The ω-conotoxin MVIIC (MVIIC) blocks N and P/Q Ca2+ channels without affecting L channels (Hillyard et al. 1992; Olivera et al. 1994; Randall & Tsien, 1995; McDonough et al. 1996, 2002; Albillos et al. 2000; see Discussion). MVIIC (3 μm) reduced current amplitude by 43 ± 4% at the same voltage (n = 7; range of inhibition 34–62%; see Fig. 2C), and contrary to the effect of GVIA, the effect of MVIIC was partially reversed after 4–6 min of washout of the toxin. Both, GVIA- and MVIIC-sensitive currents, activate at ∼−10 mV, that is, at more depolarized potentials that L-type current. Despite the broad spectrum of inhibition found for both toxins in individual cells, the significantly higher average inhibition obtained with MVIIC than with GVIA strongly suggests the existence of both, N and P/Q channels, in chemoreceptor cells. Figure 2D shows that an R or resistant calcium current is also expressed in these cells since, during simultaneous application of GVIA (1 μm), MVIIC (3 μm) and nisoldipine (1.5 μm), an important current fraction remains unblocked (42 ± 5% at +10 mV). Approximately half (47 ± 5%) of this residual current could be reversibly blocked by low concentrations of nickel (100 μm NiCl2).
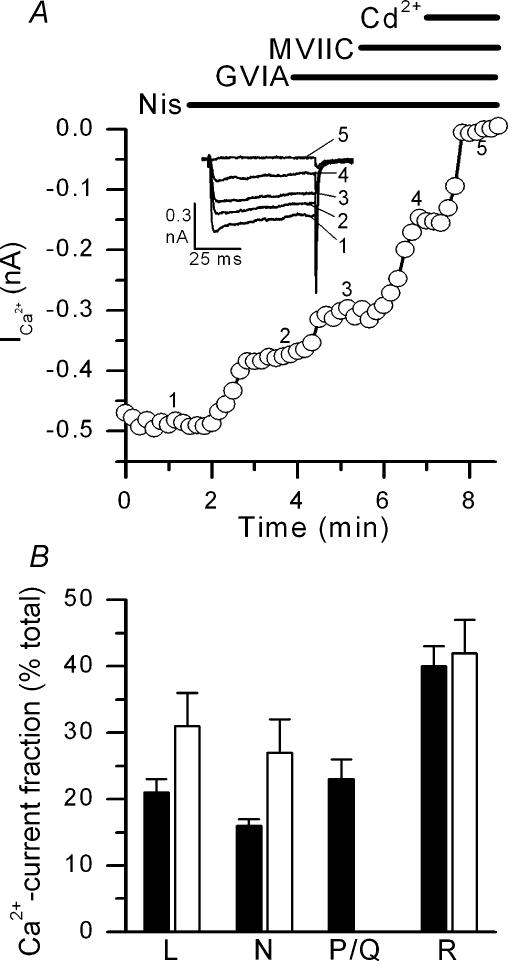
Calcium currents are modulated (sometimes in a subtype-specific way) by several factors including intra- and extracellular pH, intracellular calcium concentration and G-protein-coupled receptor pathways (Weiss & Burgoyne, 2002). The whole-cell technique of recording, by dialysing cytoplasm, may modify some of these parameters, altering the expression of calcium currents present in intact cells (Albillos et al. 2000). To approximate to this latter condition, we explored the effect of calcium channel antagonist on calcium currents recorded with the perforated-patch method. Calcium currents were evoked by application of depolarizing pulses to +10 mV from a holding potential of −80 mV every 10 s and calcium antagonists (nisoldipine, GVIA, MVIIC and Cd2+) were added to the bath solution in a cumulative way (see Fig. 3A). Figure 3B summarizes the results obtained in five cells subjected to a similar protocol. As observed in the whole-cell configuration, R-type current was predominant, representing 40 ± 3% (range 30–48%) of the total Cd2+-sensitive current; L, N and P/Q currents accounted, respectively, for 21 ± 4% (range14–27%), 16 ± 3% (range 12% and 20%) and 23 ± 6% of the total current (range 13–30%).
Figure 3. Rabbit chemoreceptor cells express L-, N-, P/Q- and R-type calcium channels.
A shows the time course of ICa recorded in perforated-patch configuration of the voltage-clamp technique in a representative cell. Currents evoked by voltage pulses to +10 mV from a holding potential of –80 mV were recorded during superfusion of the cells in control conditions and during the cumulative addition of nisoldipine (Nis; 2 μm), ω-conotoxin GVIA (GVIA; 1 μm), ω-conotoxin MVIIC (MVIIC; 3 μm) and CdCl2 (Cd2+; 200 μm) to the bath solution. Current traces obtained at the times indicated by numbers are shown in the inset. B shows fractional composition of the total calcium currents recorded at +10 mV in chemoreceptor cells (see text). Filled bars correspond to data obtained in perforated-patch recordings following the protocol shown in A. Open bars correspond to data obtained in whole-cell recordings showed in Fig. 2; the percentage of ICa carried through P/Q channels in the whole-cell recordings is not shown since it was not directly explored (see Fig. 2). n = 5–7 cells in every case.
Calcium channel types involved in the secretion of [3H]CA induced by low PO2, hypercapnic acidosis and DNP
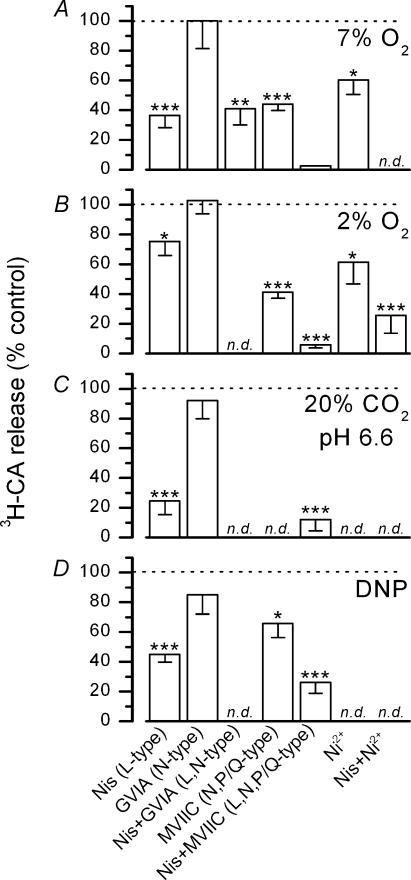
We have studied the contribution of the calcium channel subtypes present in chemoreceptor cells to the secretion of [3H]CA from CBs exposed to two intensities of hypoxic stimulation, 7% O2 and 2% O2-equilibrated solution (PO2 of ∼46 and ∼13 mmHg, respectively). Nisoldipine (2 μm) reduced the evoked release induced by 7% O2 and 2% O2 by 63 ± 8% and 25 ± 9%, respectively (see Fig. 4A and B), and this effect was not augmented by increasing nisoldipine concentration to 5 μm (data not shown). Despite its clear effect in inhibiting calcium currents, GVIA was without effect on the release of [3H]CA induced by hypoxic stimulation. Additionally, the inhibitory effect of nisoldipine on 7% O2-evoked release was not modified by simultaneous blockade of N-type current with GVIA (63% inhibition with nisoldipine alone and 59 ± 11% inhibition with nisoldipine plus GVIA). However, the evoked release of [3H]CA from CBs incubated in hypoxic conditions was significantly reduced in the presence of 3 μm MVIIC (56 ± 4% and 59 ± 4% inhibition during stimulation with 7% and 2% O2, respectively) and simultaneous application of MVIIC and nisoldipine reduced the release induced by both mild and intense hypoxia to less than a 5% of control. Low concentrations of Ni2+ (100 μm) inhibited the secretion induced by 2% O2 by 39 ± 14% and in combination with nisoldipine by 75 ± 10%, indicating that P/Q channels are sensitive to this concentration of the divalent cation.
Figure 4. Effects of different calcium channel antagonists on the release of [3H]CA induced by low, PO2 hypercapnic acidosis and DNP.
The effects of different calcium channels antagonists on the evoked release of [3H]CA is expressed as a percentage of the release obtained in the absence of the blocking agent following the experimental procedure shown in Figs 1B and C. Tested stimuli were: hypoxic solutions equilibrated with 7% O2–5% CO2–88% N2 (7% O2) or 2% O2–5% CO2–93% N2 (2% O2); hypercapnic acidosis (20% CO2, pH 6.6; solution equilibrated with 20% O2–20% CO2–60% N2 and pH 6.6); and dinitrophenol (DNP; 100 μm). Calcium antagonists were nisoldipine (Nis, 2 μm), ω-conotoxin GVIA (GVIA; 1 μm), ω-conotoxin MVIIC (MVIIC; 3 μm) and NiCl2 (Ni2+; 100 μm). n = 5–12 in both control CBs and their contralateral, drug-treated, CBs. (*P < 0.05; **P < 0.02; ***P < 0.001); n.d. experiments not done.
Figure 4C shows the sensitivity to organic calcium channel blockers of the release of [3H]CA induced by incubation of CB in an acidic solution (pH 6.6) equilibrated with 20% CO2. This secretory response was highly sensitive to nisoldipine, which at 2 μm reduced the evoked release by 75 ± 9%; conversely, the secretory response was insensitive to GVIA. During simultaneous application of nisoldipine plus MVIIC, evoked release was reduced to 12 ± 7% of that found in control, untreated CB.
Both, L and P/Q channels, mediate the secretion of [3H]CA induced by DNP (see Fig. 4D). The release response was insensitive to GVIA. However, nisoldipine and MVIIC reduced the release response by 55 ± 5% and 34 ± 9%, respectively, and when applied simultaneously, the response fell to 26 ± 7% of control. The release of [3H]CA induced by 50 μm and 200 μm DNP showed similar sensitivity to these calcium antagonists (60 ± 8% and 52 ± 3% inhibition by nisoldipine, respectively, and 85 ± 1% and 78 ± 3% inhibition by nisoldipine plus MVIIC, respectively; n = 5–6; P < 0.002 in every case).
Calcium channel types involved in the secretion of [3H]CA induced by high K+o
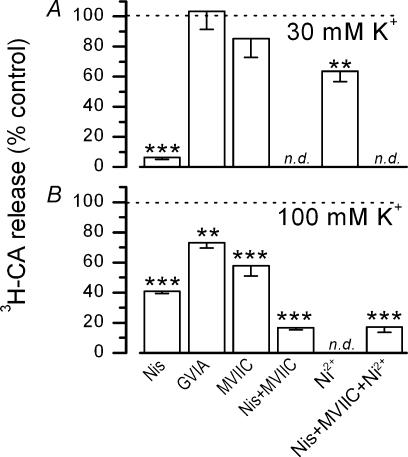
Figure 5A shows the effect of Ca2+ channel blockers on the release of [3H]CA elicited by 30 and 100 mm K+o. The response to 30 mm K+o was essentially abolished in the presence of 2 μm nisoldipine (94 ± 1% inhibition) and unaffected by GVIA (1 μm) or MVIIC (3 μm), indicating that the evoked release of [3H]CA induced by 30 mm K+o was totally mediated by calcium entry through L-type channels. 100 μm Ni2+ inhibited [3H]CA secretion by 36 ± 7%, evidencing blockade of L channels by this concentration of Ni2+. In contrast to 30 mm K+o, incubation of the CB in 100 mm K+o (see Fig. 5B), evoked a release response that was only partially sensitive to 2 μm nisoldipine (59 ± 1% inhibition). Increasing the dose of the dihydropyridine to 5 μm did not augment the degree of inhibition (61 ± 2% inhibition; n = 6 for control and experimental CBs; data not shown). Blockade of N-type channels with 1 μm GVIA reduced the release to 73 ± 4% of the control, and blockade of N- plus P/Q- type channels with 3 μm MVIIC further reduced this response to 58 ± 7%. Simultaneous application of nisoldipine plus MVIIC reduced the release to 17 ± 1% of that obtained in the control (untreated) CBs; this residual release was not affected by addition of Ni2+ (100 μm).
Figure 5. Effects of different calcium channel antagonists on the release of [3H]CA induced by 30 and 100 mM K+o.
The effect of different calcium channel antagonists on the evoked release of [3H]CA is expressed as a percentage of the release obtained in their absence, following the experimental procedure shown in Fig. 1B and C. Calcium antagonists were nisoldipine (Nis, 2 μm), ω-conotoxin GVIA (GVIA; 1 μm), ω-conotoxin MVIIC (MVIIC; 3 μm) and NiCl2 (Ni2+; 100 μm). n = 5–12 in both, control CBs and their contralateral drug-treated CBs (**P < 0.02; ***P < 0.001; n.d. experiments not done).
Effects of hypoxia, hypercapnic acidosis, DNP and high K+o on membrane potential of isolated chemoreceptor cells
The ability of inorganic and organic calcium channel antagonists to block the release of CA induced by high K+o, hypoxia, hypercapnic acidosis and DNP suggests that all four stimuli depolarize chemoreceptor cells. In addition, the differential sensitivity of those secretory responses to specific blockers of the calcium channels subtypes could reflect particular characteristics of the electrical membrane responses to the different stimuli. To investigate these proposals, we have recorded the membrane potential of isolated chemoreceptor cells subjected to different types/intensities of stimulation, using the current-clamp technique in the perforated-patch configuration. Recorded cells were identified as chemoreceptor or type I cells either by their capacity to generate action potentials or by the presence of fast inward currents under voltage-clamp conditions (Duchen et al. 1988). Some cells discharged spontaneous fast action potentials of 50–80 mV amplitude in resting conditions (extracellular solution equilibrated with 20% O2–5% CO2–75% N2; Fig. 6). In the same conditions, we also found cells that presented spontaneous depolarizing waves, slower and of lower amplitude than the action potentials (Fig. 6E). The membrane currents involved in the genesis of the spikes have not been investigated.
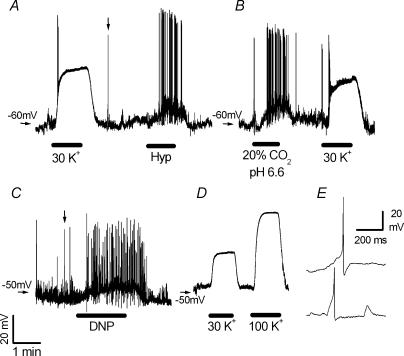
Figure 6. Effect of high K+o, low PO2, hypercapnic acidosis and dinitrophenol on membrane potential of isolated rabbit chemoreceptor cells.
A–D, recordings of membrane potential obtained in four different cells with the use of the perforated-patch technique. Superfusion solution in resting conditions was equilibrated with 20% O2–5% CO2–75% N2.Horizontal bars mark the times of application of the stimuli: hypoxia (Hyp; PO2∼25 mmHg), hypercapnic acidosis (2O% CO2, pH 6,6), 200 μm dinitrophenol (DNP) and 30 and 100 mm K+o (30 K+ and 100 K+). Calibration bars on C apply to panels A–D. E, action potentials recorded in type I cells shown on a faster time base. Upper and lower traces correspond to the spikes marked with vertical arrows in A and C, respectively.
Figure 6A shows the effect of 30 mm K+o and hypoxia on the membrane potential of an individual chemoreceptor cell. In this cell, resting membrane potential oscillated around −60 mV during superfusion with a solution equilibrated with 20% O2 (control conditions). Switching to the K+-enriched solution, induced a fast and reversible depolarization up to −20 mV. Superfusion of the cell with a solution equilibrated with 95% N2–5% CO2 (PO2 in the bath ∼25 mmHg) induced a smaller depolarization accompanied by repetitive action potential firing. Hypoxia (PO2∼20–30 mmHg) was tested in 14 cells but only eight depolarized during the hypoxic challenge; they displayed resting membrane potentials of −49.8 ± 2.6 mV, while membrane potential during hypoxia was −43.4 ± 2.5 mV with an average depolarization under low-PO2 conditions of 6.8 ± 0.5 mV.
Hypercapnic acidosis (20% CO2; pH 6.6) induced strong and consistent depolarization averaging 11.8 ± 0.7 mV in all the tested cells (n = 13). Mean resting membrane potential in these cells was −49.4 ± 2.0 mV and in acidic solution it was −36.0 ± 1.8 mV. In five cells, membrane depolarization induced by acidosis was not accompanied by discharge of an action potential. A typical response of a chemoreceptor cell during hypercapnic acidosis stimulation is shown in Fig. 6B, for easy comparison with the response induced by 30 mm K+o.
DNP was also a potent depolarizing stimulus. Mean membrane potential in 15 cells was −48.9 ± 1.7 mV in resting conditions, and this value was reduced to −37.4 ± 1.7 mV during superfusion with 200 μm DNP (Fig. 6C; average depolarization in the 15 cells was 11.5 ± 0.7 mV). Five of these cells showed repetitive firing of action potentials during DNP application. In two cells in which concentrations of 50, 100 and 200 μm were tested, it was found that depolarization induced by DNP was dose dependent (5, 7 and 12 mV change for 50, 100 and 200 μm, respectively). DNP-induced depolarization was sometimes preceded by a transient hyperpolarization, an effect that was always seen with low dose of drug (data not shown).
Figure 6D shows the voltage membrane response of a chemoreceptor cell during exposure to solutions containing 30 and 100 mm K+o. In six cells with average resting membrane potentials of −51.7 ± 2.9 mV (solution containing 5 mm K+o), 30 and 100 mm K+o brought the membrane potential to −22.5 ± 2.2 and +7.2 ± 1.4 mV, respectively. In four of these cells, one or two action potentials were discharged during the ascending phase of the high-K+-induced depolarization (see Fig. 6A and B), but no firing was observed during the steady-state depolarization.
Discussion
In neurones and secretory cells, membrane depolarization opens voltage-activated calcium channels allowing calcium to enter the cell and activate the calcium-dependent release of neurotransmitters and hormones; however, other membrane pathways, such as voltage-independent calcium channels or NCXs, and intracellular deposits may provide calcium for exocytosis (Waterman, 2000; Rizzuto, 2001). In the chemoreceptor cells of the CB, it is well established that hypoxia (Fidone et al. 1982), acidosis (Rigual et al. 1991), metabolic venoms (Obeso et al. 1992; Ortega-Saenz et al. 2003) and high K+o (Almaraz et al. 1986) induce a release of CA (mostly dopamine) that is dependent on extracellular Ca2+; however, the proposed calcium entry pathway differs depending on the intensity and type of stimulation and on the species studied (Gonzalez et al. 1994; Peers & Kemp, 2001). The data presented in this paper show that in the rabbit chemoreceptor cells, the release of CAs induced by different intensities and types of stimuli (high K+o, hypoxia, hypercapnic acidosis and DNP) is supported by the entry of calcium to the cell through voltage-activated calcium channels, mostly L and P/Q channels. In agreement with these data, we report that all these stimuli depolarize chemoreceptor cells. By comparing our data with those published on the rat CB, this work reveals that the process of chemoreception presents less dramatic differences between species than previously thought.
Up to now, information regarding the participation of voltage-dependent calcium channels in the secretion of CA from the CB is restricted to L channels. Thus, dihydropyridine antagonists almost completely block the release of CA evoked by 25–50 mm K+o and reduce, but do not abolish, that induced by different intensities of hypoxia in either the rat or rabbit CB (Shaw et al. 1989; Obeso et al. 1992; Hatton & Peers, 1997). Here we report that in rabbit chemoreceptor cells, cadmium blocks voltage-activated calcium channels, and drastically reduces to 5–20% the release of CA induced by 30 and 100 mm K+o, moderate and intense hypoxia, hypercapnic acidosis and DNP (see Fig. 1). Together, these data strongly suggest that the secretory response is secondary to the entry of calcium through voltage-dependent calcium channels and indicate that non-L channels are also coupled to neurosecretion in these cells. The observation that 74–97% of the evoked responses were also blocked by organic blockers of calcium channels supports this possibility and indicates that NCXs, which are also inhibited by cadmium (Hobai et al. 1997; Iwamoto & Shigekawa, 1998) make a minor contribution, if any, to the secretory response of chemoreceptor cells. The contribution of calcium-induced calcium release to the stimulus-evoked secretion of CA has being discounted in a previous study (Vicario et al. 2000).
Chemoreceptor cells express several types of high-voltage-gated Ca2+ channels and lack low-threshold voltage-activated Ca2+ channels. On the basis of their sensitivity to different calcium channel blockers, L-, N- P/Q- and R-type channels could be distinguished. In the perforated-patch configuration, the contribution of each current type to the total current at +10 mV was: 21% for L-type or nisoldipine-sensitive current, 16% for N-type current or GVIA-sensitive current; 23% for P/Q-type current (measured as MVIIC-sensitive current in cells pretreated with GVIA) and 40% for R-type current (measured as current resistant to simultaneous application of nisoldipine plus GVIA plus MVIIC). Although we have not performed dose–response studies, data in the literature and our findings on the release of CA in the CB indicate that the concentrations of blockers used are saturating and specific for the channel type targeted. The specific and saturating effect of 1 μm GVIA on N-type channels is well established (see Olivera et al. 1994 for review) and from a pharmacological point of view, L channels are defined by their sensitivity to dihydropyridines. In our preparation, 2 μm nisoldipine seems to be a saturating concentration for L channels, because it abolished the release of CA induced by 30 mm K+o and a higher concentration (5 μm) did not augment the inhibitory potency of the drug on hypoxia-induced release (see also Overholt & Prabhakar, 1997). The efficacy of MVIIC in blocking P/Q currents in our experimental conditions is more debatable. Although the concentration used in our experiments (3 μm) is saturating for blocking P/Q (and also N) channels (Hillyard et al. 1992; Olivera et al. 1994; Randall & Tsien, 1995; McDonough et al. 1996, Albillos et al. 2000; 2002), in some preparations, blockade develops very slowly (20–30 min); yet, in chemoreceptor cells (see Fig. 3), as is the case in peripheral neurones (McDonough et al. 1996, 2002), the effect of MVIIC is fast, and completes in less than 1.5 min, indicating that both P and Q types are fully blocked in our experimental conditions.
R-type channel expression in rabbit chemoreceptor cells is in the high range of percentages found for resistant currents in other excitable cells (Randall & Tsien, 1995; Albillos et al. 2000; Wilson et al. 2000) including rat chemoreceptor cells (Silva & Lewis, 1995), and has similar magnitude in both whole-cell and perforated-patch recording conditions. This latter finding evidences the existence of important differences in the regulatory mechanisms of resistant currents expressed in CB chemoreceptor cells versus chromaffin cells, since, in the latter, R currents are only recorded under perforated-patch configuration (Albillos et al. 2000). As is the case in rat chemoreceptor cells (Silva & Lewis, 1995), R-type current in rabbit chemoreceptor cells was non-inactivating, abolished by Cd2+ (200 μm) and significantly reduced by low concentration of Ni2+ (see Fig. 2D). In the experiments performed in whole-cell configuration (in which a complete I–V relationship was obtained in control conditions and during drug application), the average values for the different drug-sensitive currents were similar or slightly superior to those obtained in the perforated-patch configuration; inclusion in the drug-sensitive fraction of the spontaneous rundown of the current (which is variable from cell to cell and faster in the whole-cell configuration), and the variability among different cells in the expression of a particular type of channel may explain those differences.
Our findings on ICa types in chemoreceptor cells of the rabbit CB are in general agreement with those reported by Overholt & Prabhakar (1997) using Ba2+, instead of Ca2+, as a charge carrier. These authors conclude that the P/Q channels expressed in these cells belong, from a pharmacological point of view, to the P subtype since 100 nmω-agatoxin IVa (Aga IVa) blocked 19% of total current and MVIIC (1 μm) was without effect when applied in presence of a cocktail containing nisoldipine + GVIA + Aga IVa. We have not explored this possibility in detail, but data on the release of CAs suggest that chemoreceptor cells may express both, P- and Q-type channels; thus, while nisoldipine plus MVIIC abolishes the secretion of CA induced by 2% O2 (see Fig. 4), a mixture of 2 μm nisoldipine, 1 μm GVIA and 100 nmω-agatoxin-TK (a toxin that exhibits similar potency and calcium-channel specificity to that of Aga IVa; Teramoto et al. 1995) only could reduce this secretory response to 42% (Rocher, Gonzalez and Almaraz, unpublished data).
We found that voltage-dependent calcium channels mediate the bulk release of CA evoked by high K+o, mild and strong hypoxia, hypercapnic acidosis and DNP. Although these data indicate that membrane depolarization is the trigger for the evoked secretory responses, the literature lacks data about the effects of CB stimulants on the membrane potential of rabbit chemoreceptor cells supporting this possibility. Here we report that all the above stimuli depolarize rabbit chemoreceptor cells and therefore that they are able to activate calcium entry through voltage-dependent calcium channels. Different contributions of L and non-L calcium channels to the secretory responses induced by the stimulants is a common finding in the literature. Among the factors responsible for those differences, the voltage activation threshold of the implied channels is an important one. In chemoreceptor cells, nisoldipine-sensitive current of chemoreceptor cells activates at membrane potentials significantly more negative than the other current populations (−30 versus−10 mV; see Fig. 2), indicating that mild membrane depolarizations will activate only L-type channels while stronger ones will be necessary to induce calcium entry through non-L channels. We found that extracellular medium containing 30 mm K+o fixed the membrane potential at −22 mV and induced a release of CA that could be abolished by nisoldipine alone, while hypoxia, hypercapnic acidosis and DNP (that brought the membrane potential to values positive to 0 mV during action potential firing) or 100 mm K+o (that fixed the membrane potential at +7 mV), induced a secretory response mediated by both L and non-L currents. The precise correlation between the pattern of electrical activity and the nature of the ICa activated by each specific stimulus would require the measurement of the intracellular Ca2+ response elicited by each stimulus and its sensitivity to specific Ca2+ channel antagonists, a project outside the scope of the present study. However, an attempt to correlate the pattern of the electrical activity and the identity of the ICa induced by the different stimuli can be made on the basis of the sensitivity of the CA release response to the different specific Ca2+ channel antagonists. Based on that, it appears that sustained depolarization of moderate intensity favours the participation of L-type channels, because the release response elicited by 30 mm K+o is fully blocked by nisoldipine. On the other hand, spiking behaviour seems to favour the participation of other subsets of Ca2+ channels, however, there is not a well defined correlation. We believe that the absence of a clear correlation in this regard is due to the fact that each stimulus, in addition to the electrical response, produces other effects that are stimulus specific and, probably, capable of modifying calcium homeostasis (through effects on specific Ca2+ channels or intracellular calcium buffering capacity) and/or the sensitivity of the exocytotic machinery to Ca2+ (Montoro et al. 1996; Duchen & Biscoe, 1992; Summers et al. 2000, 2002; Rizzuto, 2001; Ahdut-Hacohen et al. 2004). In this regard, the most obvious example is the low secretory potency of hypercapnic acidosis (and also of acidosis alone, see Rigual et al. 1991) in comparison to that of hypoxia and DNP, yet the three stimuli produce a comparable spiking activity (Buckler & Vaughan-Jones, 1994a, 1994b; see Fig. 6 in this paper). Other factors such as location and inactivation characteristics of the different types of calcium channels expressed in chemoreceptor cells could also determine the relative contribution of each channel population to the observed release response (see below).
Despite chemoreceptor cells expressing L-, N-, P/Q- and R-type Ca2+ channels, only L and P/Q channels are involved in the secretion of CA evoked by 30 mm K+o, hypoxia, hypercapnic acidosis and DNP. The presence of Ca2+ channels not coupled to the exocytotic machinery is a common finding in other secreting cells. It has been suggested that the mouth of the channels could be located distant from the active zones of the membrane where vesicular release takes place (Artalejo et al. 1994; Lopez et al. 1994; Lara et al. 1998; Mansvelder & Kits, 2000; Waterman, 2000). Differing from our findings, Kim et al. (2001) found that 85% of the hypoxic release of substance P from the CB is mediated by calcium entry via N-type channels. Storage of CA and substance P (SP) in separate populations of vesicles coupled to different types of Ca2+ channels might explain the discrepant findings. This mechanism has been proposed in the autonomic nerves innervating the urinary bladder to explain why the release of ATP is not linked to N-type channels, while that of acetylcholine is highly sensitive to GVIA (Waterman, 2000). Additionally, the possibility exists that most of the SP released in the experiments of Kim et al. (2001) comes from intraglomic sensory nerve endings synaptically activated during hypoxia, because the presence of substance P in rabbit chemoreceptor cells is not firmly established (Kusakabe et al. 1994versusKim et al. 2001; see also Gauda, 2002), while there is general agreement on the presence of SP-positive sensory fibres in all studied species (Gauda, 2002). However, N-type channels do participate (together with L and P/Q channels) in the secretion of CA induced by 100 mm K+o (see Fig. 5); in these conditions, it is possible that very high calcium levels far away from catecholamine-active zones elevates calcium concentration in these microdomains by passive diffusion. R-type channels do not seem to contribute significantly to the release of CA either, although 100 μm Ni2+ (which inhibits a fraction of R-type current, see Fig. 2D) reduces significantly the release evoked by hypoxia and 30 mm K+; this effect was reproduced by nisoldipine alone or in combination with MVIIC and therefore should result from simultaneous blockade of L- and P/Q-type channels (Hobai et al. 2000; N'Gouemo & Rittenhouse, 2000). We can not discount the possiblility however, that the residual release of CA evoked by 100 mm K+o after blockade of L-, N-, P/Q- and Ni2+-sensitive R-type channels (17% of the release that was sensitive to Cd2+; compare Figs 1C and 5B) is mediated by R current resistant to 100 μm Ni2+ (Tottene et al. 2000). It might be argued that the lack of participation of N- and/or R-type Ca2+ channels in the release of CA from chemoreceptor cells could be due to preferential inactivation of these channel types during the long-lasting stimulation period (10 min) of our release experiments. We do not think this is the case for N channels because, as pointed out above, Ca2+ entering trough N-type Ca2+ channels mediates the bulk release of substance P from the CB in response to long-lasting stimulus (>10 min; Kim et al. 2001). With our experimental approach we can not discount, however, that R channels participate in the initial fast secretory response that takes place during the first millisecond of stimulation, as has been described in mouse adrenal slices (Albillos et al. 2000). Even if it is the case in chemoreceptor cells, it should be remembered that the CB is called to respond to long-lasting stimulation periods comparable to or even longer than our 10 min stimuli. Thus, in this regard, our release experiments provide information on the behaviour of the CA release process that mimics the physiological situation.
Finally, it should be mentioned here that, in previous work, we concluded that L-type channels do not participate in the release of CA induced by acidosis and DNP. Specifically, we reported insensitivity of the DNP-evoked release to nisoldipine and insensitivity of the acidic response to Bay K8644 (Rocher et al. 1991; Obeso et al. 1992). While in the first case, the lack of response could be due to the use of a non-saturating dose of nisoldipine (625 nm; see Overholt & Prabhakar, 1997), we have no data to explain the latter observation. The possibility exists that the mechanisms mediating the action of Bay K8644 (Armstrong & Eckert, 1987; Borges et al. 2002; Erxleben et al. 2003) are directly or indirectly sensitive to changes in pH/PCO2.
Our understanding of the chemoreception process in the CB has undergone significant growth in the last two decades, but the reported differences between species have made it difficult to construct a general frame for chemosensory transduction. For example, different K+ channels seem to mediate CB responses to hypoxia in rat (Buckler, 1997; Buckler et al. 2000; Peers & Kemp, 2001), rabbit (Lopez-Barneo et al. 1988; Lopez-Lopez et al. 1989), and cat (Chou & Shirahata, 1996). Additionally, the different experimental approaches used to explore the transduction pathways for acid and DNP stimuli in rat versus rabbit CB have generated two chemotransduction hypotheses for these stimuli (Rocher et al. 1991versusBuckler & Vaughan-Jones, 1998). In rat chemoreceptor cells, measurements of intracellular calcium and electrophysiological techniques evidenced that both stimuli produce membrane depolarization through the inhibition of a background K+ current and augmented cytosolic Ca2+ through a mechanism blocked by 2 mm Ni2+ and partially sensitive to dihydropyridines (Buckler & Vaughan-Jones, 1994b; Buckler & Vaughan-Jones, 1998; Buckler et al. 2000). This sequence of events resembles that found during low PO2 stimulation in the same specie (Buckler et al. 2000). In the rabbit CB, where the release of CA during acidic/DNP stimulation was characterized, it was proposed that intracellular acidosis induced by these stimuli releases CA by sequential activation of Na+/H+ and Na+/Ca2+ exchangers (Rocher et al. 1991). In the present work we have studied for the first time the electrical membrane responses of rabbit chemoreceptor cells during stimulation with acid and DNP. Here, we report that hypercapnic acidosis and DNP also depolarize chemoreceptor cells in the rabbit CB and that the bulk of the release of CA induced by these stimuli is dependent on activation of L and P/Q calcium channels. These results refute the previous hypothesis, and suggest that the chemotransduction pathway for these stimuli is similar in both species. Further investigation directed to exploring the existence in rabbit CB chemoreceptor cells of those K+ channels inhibited by chemostimulants in rat chemoreceptor cells (calcium-dependent IK and two-pore domain K+ channels) is necessary to elicit the real differences in chemotransduction pathways between both species.
Acknowledgments
We wish to thank Maria de los Llanos Bravo, Rosa García Velasco and Alfonso Pérez Vegara for excellent technical assistance. This work was supported by Spanish DGICYT Grants BFI2001-1713, BFI2002-03467 and BFI2003/1627. Support was also obtained from Red Respira-Separ (Instituto Carlos III; FISS) and Junta de Castilla y Leon, Grants VA 08/03, VA 092/03 and VA045/04.
References
- Ahdut-Hacohen R, Duridanova D, Meiri H, Rahamimoff R. Hydrogen ions control synaptic vesicle ion channel activity in Torpedo electromotor neurones. J Physiol. 2004;556:347–352. doi: 10.1113/jphysiol.2003.058818. 10.1113/jphysiol.2003.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albillos A, Neher E, Moser T. R-Type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J Neurosci. 2000;20:8323–8330. doi: 10.1523/JNEUROSCI.20-22-08323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaraz L, Gonzalez C, Obeso A. Effects of high potassium on the release of [3H]dopamine from the cat carotid body in vitro. J Physiol. 1986;379:293–307. doi: 10.1113/jphysiol.1986.sp016254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D, Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987;84:2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. doi: 10.1038/367072a0. 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- Borges R, Machado JD, Betancor G, Camacho M. Pharmacological regulation of the late steps of exocytosis. Ann NY Acad Sci. 2002;971:184–192. doi: 10.1111/j.1749-6632.2002.tb04462.x. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol. 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994a;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. J Physiol. 1994b;478:157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol. 1998;513:819–833. doi: 10.1111/j.1469-7793.1998.819ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CL, Shirahata M. Two types of voltage-gated K channels in carotid body cells of adult cats. Brain Res. 1996;742:34–42. doi: 10.1016/s0006-8993(96)00987-0. [DOI] [PubMed] [Google Scholar]
- Duchen MR, Biscoe TJ. Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol. 1992;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Caddy KW, Kirby GC, Patterson DL, Ponte J, Biscoe TJ. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience. 1988;26:291–311. doi: 10.1016/0306-4522(88)90146-7. 10.1016/0306-4522(88)90146-7. [DOI] [PubMed] [Google Scholar]
- Erxleben C, Gomez-Alegria C, Darden T, Mori Y, Birnbaumer L, Armstrong DL. Modulation of cardiac Cav 1.2 channels by dihydropyridine and phosphatase inhibitor requires Ser-1142 in the domain III pore loop. Proc Natl Acad Sci U S A. 2003;100:2929–2934. doi: 10.1073/pnas.2628046100. 10.1073/pnas.2628046100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidone S, Gonzalez C, Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber LA, McCleskey EW. L-type calcium channels in type I cells of the rat carotid body. J Neurophysiol. 1993;70:1378–1384. doi: 10.1152/jn.1993.70.4.1378. [DOI] [PubMed] [Google Scholar]
- Gauda EB. Gene expression in peripheral arterial chemoreceptors. Microsc Res Techn. 2002;59:153–167. doi: 10.1002/jemt.10190. 10.1002/jemt.10190. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Peers C. Electrochemical detection of K+-evoked quantal secretory events from isolated rat type I carotid body cells. Exp Physiol. 1997;82:415–418. doi: 10.1113/expphysiol.1997.sp004036. [DOI] [PubMed] [Google Scholar]
- Hillyard DR, Monje VD, Mintz IM, Bean BP, Nadasdi L, Ramachandran J, et al. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992;9:69–77. doi: 10.1016/0896-6273(92)90221-x. 10.1016/0896-6273(92)90221-X. [DOI] [PubMed] [Google Scholar]
- Hobai IA, Bates JA, Howarth FC, Levi AJ. Inhibition by external Cd2+ of Na/Ca exchange and L-type Ca channel in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 1997;272:H2164–H2172. doi: 10.1152/ajpheart.1997.272.5.H2164. [DOI] [PubMed] [Google Scholar]
- Hobai IA, Hancox JC, Levi AJ. Inhibition by nickel of the L-type Ca channel in guinea pig ventricular myocytes and effect of internal cAMP. Am J Physiol Heart Circ Physiol. 2000;279:H692–H701. doi: 10.1152/ajpheart.2000.279.2.H692. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Shigekawa M. Differential inhibition of Na+/Ca2+ exchanger isoforms by divalent cations and isothiourea derivative. Am J Physiol Cell Physiol. 1998;275:C423–C430. doi: 10.1152/ajpcell.1998.275.2.C423. [DOI] [PubMed] [Google Scholar]
- Jiang RG, Eyzaguirre C. Effects of hypoxia and putative transmitters on [Ca2+]i of rat glomus cells. Brain Res. 2004;995:285–296. doi: 10.1016/j.brainres.2003.09.075. 10.1016/j.brainres.2003.09.075. [DOI] [PubMed] [Google Scholar]
- Kim DK, Oh EK, Summers BA, Prabhakar NR, Kumar GK. Release of substance P by low oxygen in the rabbit carotid body: evidence for the involvement of calcium channels. Brain Res. 2001;892:359–369. doi: 10.1016/s0006-8993(00)03272-8. 10.1016/S0006-8993(00)03272-8. [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Kawakami T, Tanabe Y, Fujii S, Takenaka T. Distribution of substance P-containing and catecholaminergic nerve fibers in the rabbit carotid body: an immunohistochemical study in combination with catecholamine fluorescent histochemistry. Arch Histol Cytol. 1994;57:193–199. doi: 10.1679/aohc.57.193. [DOI] [PubMed] [Google Scholar]
- Lara B, Gandia L, Martinez-Sierra R, Torres A, Garcia AG. Q-type Ca2+ channels are located closer to secretory sites than L-type channels: functional evidence in chromaffin cells. Pflugers Arch. 1998;435:472–478. doi: 10.1007/s004240050541. 10.1007/s004240050541. [DOI] [PubMed] [Google Scholar]
- Lopez MG, Villarroya M, Lara B, Martinez Sierra R, Albillos A, Garcia AG, et al. Q- and L-type Ca2+ channels dominate the control of secretion in bovine chromaffin cells. FEBS Lett. 1994;349:331–337. doi: 10.1016/0014-5793(94)00696-2. 10.1016/0014-5793(94)00696-2. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Lopez-Lopez JR, Ureña J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez J, Gonzalez C, Ureña J, Lopez-Baneo J. Low PO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989;93:1001–1015. doi: 10.1085/jgp.93.5.1001. 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough SI, Boland LM, Mintz IM, Bean BP. Interactions among toxins that inhibit N-type and P-type calcium channels. J Gen Physiol. 2002;119:313–328. doi: 10.1085/jgp.20028560. 10.1085/jgp.20028560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough SI, Swartz KJ, Mintz IM, Boland LM, Bean BP. Inhibition of calcium channels in rat central and peripheral neurons by omega-conotoxin MVIIC. J Neurosci. 1996;16:2612–2623. doi: 10.1523/JNEUROSCI.16-08-02612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Kits KS. Calcium channels and the release of large dense core vesicles from neuroendocrine cells: spatial organization and functional coupling. Prog Neurobiol. 2000;62:427–441. doi: 10.1016/s0301-0082(00)00003-4. 10.1016/S0301-0082(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. 10.1016/0896-6273(92)90223-Z. [DOI] [PubMed] [Google Scholar]
- Montoro RJ, Ureña J, Fernandez-Chacon R, Alvarez de Toledo G, Lopez-Barneo J. Oxygen sensing by ion channels and chemotransduction in single glomus cells. J Gen Physiol. 1996;107:133–143. doi: 10.1085/jgp.107.1.133. 10.1085/jgp.107.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Gouemo P, Rittenhouse AR. Biophysical and pharmacological characterization of voltage-sensitive calcium currents in neonatal rat inferior colliculus neurons. Neuroscience. 2000;96:753–765. doi: 10.1016/s0306-4522(00)00006-3. 10.1016/S0306-4522(00)00006-3. [DOI] [PubMed] [Google Scholar]
- Obeso A, Rocher A, Fidone S, Gonzalez C. The role of dihydropyridine-sensitive Ca2+ channels in stimulus-evoked catecholamine release from chemoreceptor cells of the carotid body. Neuroscience. 1992;47:463–472. doi: 10.1016/0306-4522(92)90260-9. 10.1016/0306-4522(92)90260-9. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Miljanich GP, Ramachandran J, Adams ME. Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- Ortega-Saenz P, Pardal R, Garcia-Fernandez M, Lopez-Barneo J. Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J Physiol. 2003;548:789–800. doi: 10.1113/jphysiol.2003.039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholt JL, Prabhakar NR. Ca2+ current in rabbit carotid body glomus cells is conducted by multiple types of high-voltage-activated Ca2+ channels. J Neurophysiol. 1997;78:467–474. doi: 10.1152/jn.1997.78.5.2467. [DOI] [PubMed] [Google Scholar]
- Peers C, Carpenter E, Hatton CJ, Wyatt CN, Bee D. Ca2+ channel currents in type I carotid body cells of normoxic and chronically hypoxic neonatal rats. Brain Res. 1996;739:251–257. doi: 10.1016/s0006-8993(96)00832-3. 10.1016/S0006-8993(96)00832-3. [DOI] [PubMed] [Google Scholar]
- Peers C, Kemp PJ. Acute oxygen sensing: diverse but convergent mechanisms in airway and arterial chemoreceptors. Respir Res. 2001;2:145–149. doi: 10.1186/rr51. 10.1186/rr51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigual R, Lopez-Lopez JR, Gonzalez C. Release of dopamine and chemoreceptor discharge induced by low pH and high PCO2 stimulation of the cat carotid body. J Physiol. 1991;433:519–531. doi: 10.1113/jphysiol.1991.sp018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R. Intracellular Ca2+ pools in neuronal signalling. Curr Opin Neurobiol. 2001;11:306–311. doi: 10.1016/s0959-4388(00)00212-9. 10.1016/S0959-4388(00)00212-9. [DOI] [PubMed] [Google Scholar]
- Rocher A, Geijo E, Caceres AI, Gonzalez C, Almaraz L. A reevaluation of the mechanisms involved in the secretion of catecholamine evoked by 2,4-dinitrophenol from chemoreceptor cells of the rabbit carotid body. Adv Exp Med Biol. 2003;536:85–93. doi: 10.1007/978-1-4419-9280-2_11. [DOI] [PubMed] [Google Scholar]
- Rocher A, Gonzalez C, Almaraz L. Adenosine inhibits L-type Ca2+ current and catecholamine release in the rabbit carotid body chemoreceptor cells. Eur J Neurosci. 1999;11:673–681. doi: 10.1046/j.1460-9568.1999.00470.x. 10.1046/j.1460-9568.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- Rocher A, Obeso A, Gonzalez C, Herreros B. Ionic mechanisms for the transduction of acidic stimuli in rabbit carotid body glomus cells . J Physiol. 1991;433:533–548. doi: 10.1113/jphysiol.1991.sp018442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K, Montague W, Pallot DJ. Biochemical studies on the release of catecholamines from the rat carotid body in vitro. Biochim Biophys Acta. 1989;1013:42–46. doi: 10.1016/0167-4889(89)90125-0. 10.1016/0167-4889(89)90125-0. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Lewis DL. L- and N-type Ca2+ channels in adult rat carotid body chemoreceptor type I cells. J Physiol. 1995;489:689–699. doi: 10.1113/jphysiol.1995.sp021083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers BA, Overholt JL, Prabhakar NR. Augmentation of L-type calcium current by hypoxia in rabbit carotid body glomus cells: evidence for a PKC-sensitive pathway. J Neurophysiol. 2000;84:1636–1644. doi: 10.1152/jn.2000.84.3.1636. [DOI] [PubMed] [Google Scholar]
- Summers BA, Overholt JL, Prabhakar NR. CO2 and pH independently modulate L-type Ca2+ current in rabbit carotid body glomus cells. J Neurophysiol. 2002;88:604–612. doi: 10.1152/jn.2002.88.2.604. [DOI] [PubMed] [Google Scholar]
- Teramoto T, Niidome T, Miyagawa T, Nishizawa Y, Katayama K, Sawada K. Two types of calcium channels sensitive to omega-agatoxin-TK in cultured rat hippocampal neurones. Neuroreport. 1995;6:1684–1688. doi: 10.1097/00001756-199508000-00022. [DOI] [PubMed] [Google Scholar]
- Tottene A, Volsen S, Pietrobon D. Alpha1E subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña J, Fernandez-Chacon R, Benot AR, Alvarez de Toledo GA, Lopez-Barneo J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc Natl Acad Sci U S A. 1994;91:10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario I, Obeso A, Rocher A, Lopez-Lopez JR, Gonzalez C. Intracellular Ca2+ stores in chemoreceptor cells of the rabbit carotid body: significance for chemoreception. Am J Physiol Cell Physiol. 2000;279:C51–C61. doi: 10.1152/ajpcell.2000.279.1.C51. [DOI] [PubMed] [Google Scholar]
- Wakamori M, Strobeck M, Niidome T, Teramoto T, Imoto K, Mori Y. Functional characterization of ion permeation pathway in the N-type Ca2+ channel. J Neurophysiol. 1998;79:622–634. doi: 10.1152/jn.1998.79.2.622. [DOI] [PubMed] [Google Scholar]
- Waterman SA. Voltage-gated calcium channels in autonomic neuroeffector transmission. Prog Neurobiol. 2000;60:181–210. doi: 10.1016/s0301-0082(99)00025-8. 10.1016/S0301-0082(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Weiss JL, Burgoyne RD. Sense and sensibility in the regulation of voltage-gated Ca2+ channels. Trends Neurosci. 2002;25:489–491. doi: 10.1016/s0166-2236(02)02247-6. 10.1016/S0166-2236(02)02247-6. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Toth PT, Oh SB, Gillard SE, Volsen S, Ren D, et al. The status of voltage-dependent calcium channels in alpha 1E knock-out mice. J Neurosci. 2000;20:8566–8571. doi: 10.1523/JNEUROSCI.20-23-08566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]