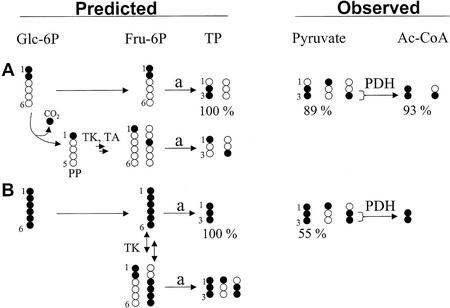
Figure 3.
Predicted and observed isotopomer pattern in triose-P (TP) and derivatives (pyruvate and acetyl-CoA) after feeding of 13C-labeled Glc. Developing embryos were labeled with [1,2-13C2]Glc (A) or [U-13C6]Glc (B) in SMA medium (10% enrichment of labeled Glc). Fragments of Ala and C18:1(1-2) were measured by GC/MS, representing pyruvate and acetyl-CoA, respectively. For the sake of clarity, the figure does not show all expected and found isotopomers. Fru-6P is derived from Glc or by reactions of the OPPP. After glycolytic cleavage of Fru-6P (a), dihydroxyacetone phosphate and glyceraldehyde 3-phosphate are assumed to be in isotopic equilibrium (triose-P [TP]). A, By sole glycolysis, the abundance of 13C2 label from [1,2-13C2]Glc is retained in triose-P, whereas the OPPP reduces the abundance of 13C2-labeled triose-P. Nearly 100% of possible 13C2 abundance was found for pyruvate and acetyl-CoA. B, By sole glycolysis, [U-13C6]Glc is transformed to [U-13C3]pyruvate (100%). By involvement of transketolase (TK) and transaldolase (TA) in synthesis of Fru-6P, the abundance of [U-13C3]pyruvate is reduced and mainly [1-13C]- and [2,3-13C2]pyruvate are formed. Because the contribution of the oxidative part of the OPPP is very low (A), the observed labeling pattern from [U-13C6]Glc can be explained by reversible exchange of C1/C2 of Fru-6P by transketolase. Due to highly reversible reactions of the OPPP, an exact determination of fluxes requires the measurement of fractional labeling in intermediates of the OPPP (see e.g. Roscher et al., 2000). PDH, Pyruvate dehydrogenase complex; PP, pentose-phosphate.