Abstract
1. Recovery of 3H-dopamine and 3H-L-dopa infused intra-arterially at a constant rate of 20 ng/min into the cat spleen perfused with Krebs bicarbonate solution was 52·6±3·8% and 90·5±3·1% respectively. Recovery of 3H-dopamine in spleens treated with α-methyl-p-tyrosine was 67·7±6·7% and was not significantly different from untreated spleens.
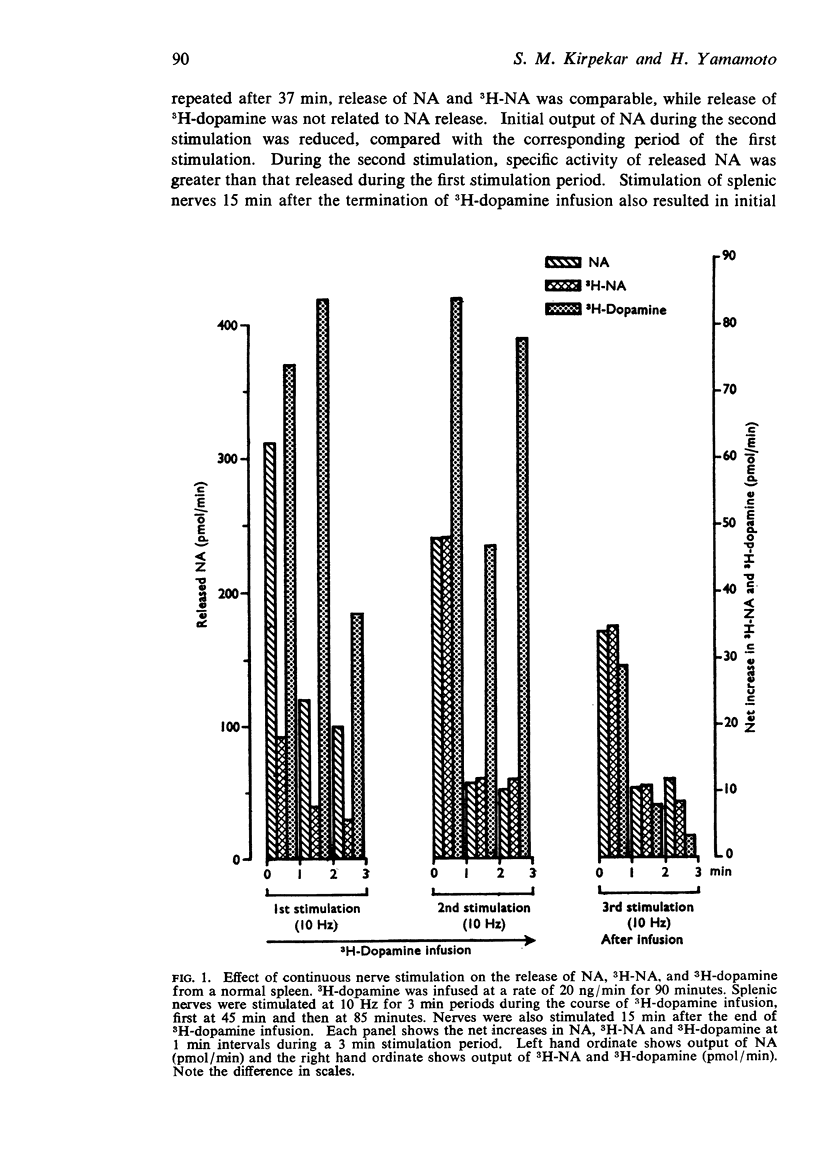
2. Release of 3H-dopamine by nerve stimulation after an infusion of 3H-dopamine resembled release of endogenous noradrenaline. Release of 3H-L-dopa and 3H-L-tyrosine after their infusion did not occur in any consistent manner by nerve stimulation.
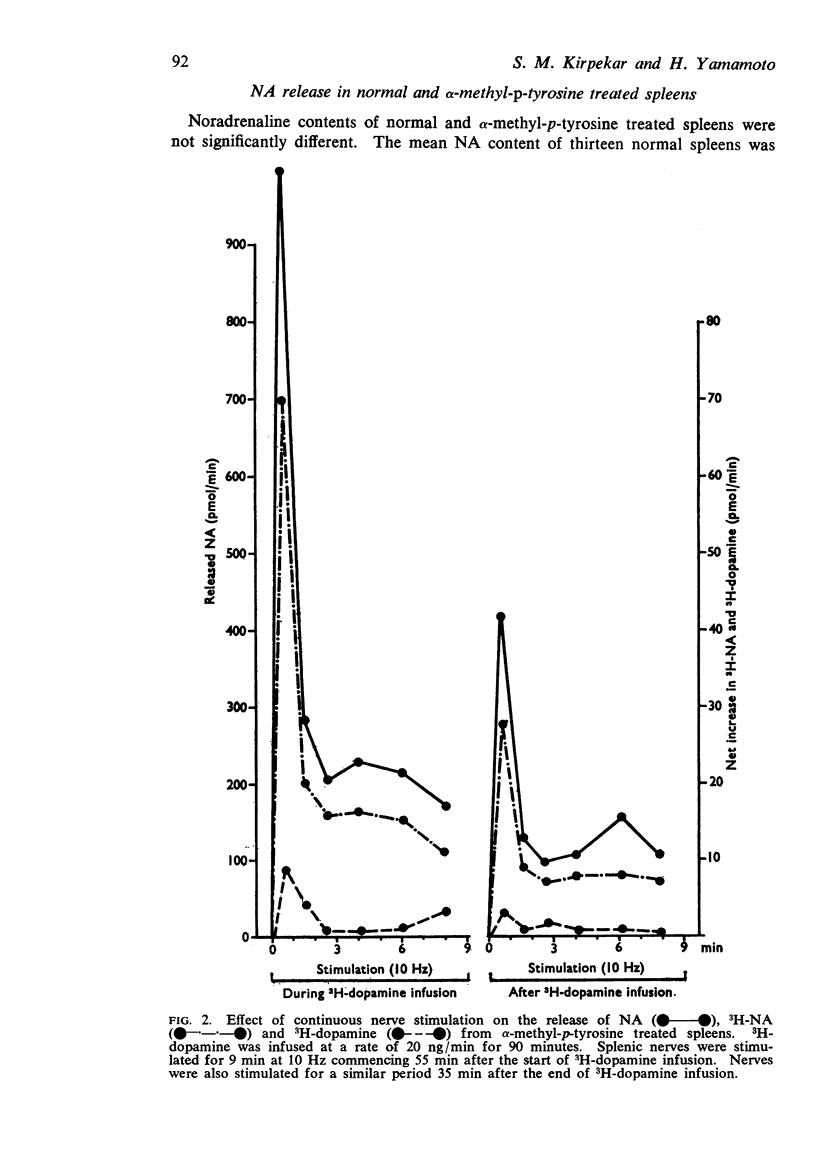
3. Preferential release of 3H-noradrenaline formed from 3H-dopamine was not observed during continuous nerve stimulation. Specific activity of released 3H-noradrenaline remained constant during any single stimulation period with or without 3H-dopamine infusion. Treating the cats with α-methyl-p-tyrosine did not change the time course of 3H-noradrenaline release.
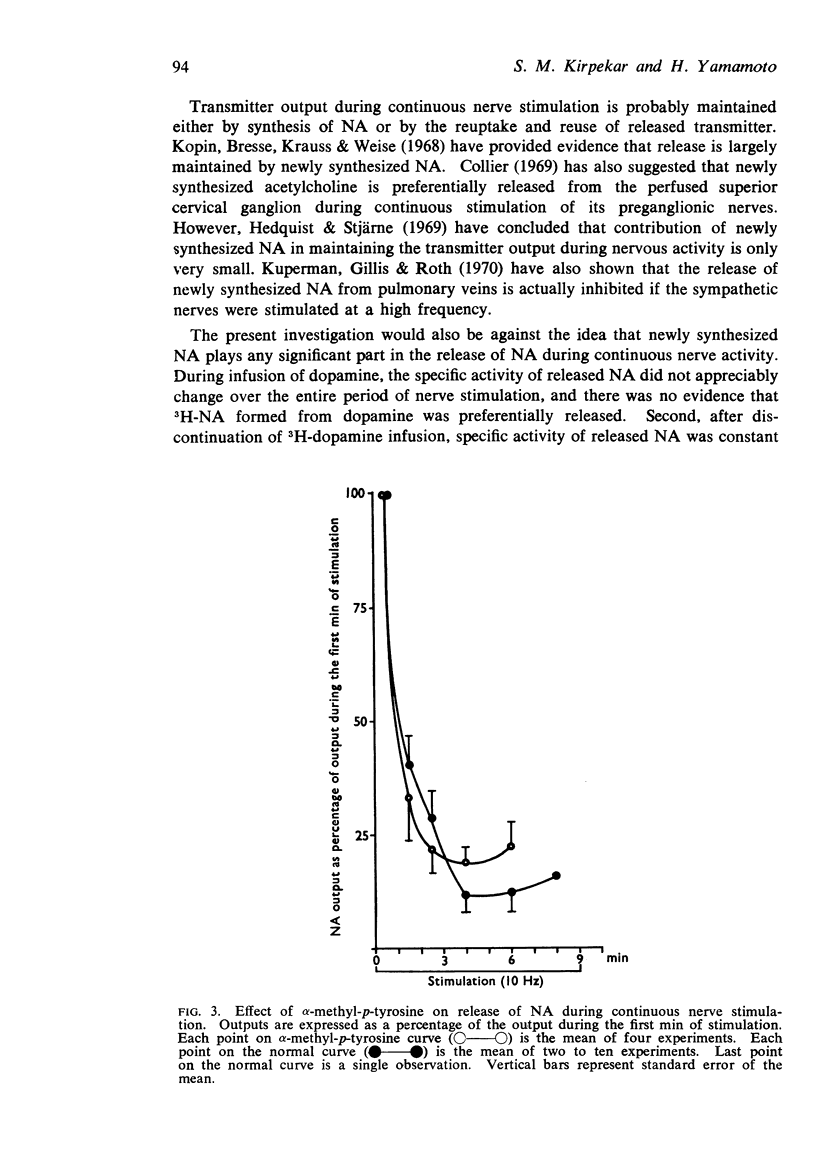
4. Output of noradrenaline expressed as a percentage of the first minute output in both normal and α-methyl-p-tyrosine treated spleens was not significantly different at various times during continuous nerve stimulation.
5. Specific activity of released noradrenaline formed from 3H-dopamine was always greater than the specific activity of the spleen in normal spleens and spleens treated with α-methyl-p-tyrosine.
6. It is concluded that newly synthesized or infused noradrenaline initially mixes with a more rapidly turning pool and only slowly with the entire tissue store. During continuous nerve stimulation there is no further preferential release of newly synthesized noradrenaline. Released noradrenaline truly represents the state of the releasable pool and will vary with the latter.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- BROWN G. L., GILLESPIE J. S. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957 Aug 29;138(1):81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIDSEY C. A., HARRISON D. C. Studies on the distribution of exogenous norepinephrine in the sympathetic neurotransmitter store. J Pharmacol Exp Ther. 1963 May;140:217–223. [PubMed] [Google Scholar]
- Collier B. The preferential release of newly synthesized transmitter by a sympathetic ganglion. J Physiol. 1969 Nov;205(2):341–352. doi: 10.1113/jphysiol.1969.sp008969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley D. P., Geffen L. B. Effect of nerve stimulation on the noradrenaline content of the spleen. Proc R Soc Lond B Biol Sci. 1966 Dec 13;166(1004):303–315. doi: 10.1098/rspb.1966.0101. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Kirpekar S. M. The uptake and release of radioactive noradrenaline by the splenic nerves of cats. J Physiol. 1966 Nov;187(1):51–68. doi: 10.1113/jphysiol.1966.sp008075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedqvist P., Stjärne L. The relative role of recapture and of de novo synthesis for the maintenance of neurotransmitter homeostasis in noradrenergic nerves. Acta Physiol Scand. 1969 Jul;76(3):270–283. doi: 10.1111/j.1748-1716.1969.tb04470.x. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin I. J., Breese G. R., Krauss K. R., Weise V. K. Selective release of newly synthesized norepinephrine from the cat spleen during sympathetic nerve stimulation. J Pharmacol Exp Ther. 1968 Jun;161(2):271–278. [PubMed] [Google Scholar]
- Kupferman A., Gillis C. N. Roth RH: Influence of sympathetic nerve stimulation on conversion of H3-tyrosine to H3-catecholamine and on H3-norepinephrine disposition in rabbit pulmonary artery. J Pharmacol Exp Ther. 1970 Feb;171(2):214–222. [PubMed] [Google Scholar]
- LEVITT M., SPECTOR S., SJOERDSMA A., UDENFRIEND S. ELUCIDATION OF THE RATE-LIMITING STEP IN NOREPINEPHRINE BIOSYNTHESIS IN THE PERFUSED GUINEA-PIG HEART. J Pharmacol Exp Ther. 1965 Apr;148:1–8. [PubMed] [Google Scholar]


