Table 2.
Total urea synthesis and the transfer of 15N from [5-15N]glutamine to urea in control subjects and patients with disorders of the urea cycle of varying severity
| Subject category | Urea flux, μmol/(kg⋅h) | Glutamine flux, μmol/(kg⋅h) | 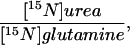 |
Transfer of glutamine to urea, percent dose |
|---|---|---|---|---|
 |
||||
| Healthy controls | 131 ± 21 | 380 ± 63 | 0.42 ± 0.06 | 27.9 ± 7.2 |
| Null patients | 82 ± 17‡ | 456 ± 103§ | 0.003 ± 0.007*** | −0.2 ± 1.2*** |
| Partial OTC males | 97 ± 45* | 454 ± 51 | 0.16 ± 0.04*** | 6.8 ± 2.4*** |
| Symptomatic OTC females | 108 ± 9 | 442 ± 68 | 0.18 ± 0.01*** | 9.3 ± 1.8*** |
| Asymptomatic OTC females | 99 ± 27* | 380 ± 89 | 0.26 ± 0.04***,† | 12.6 ± 2.8***,† |
| ASLD heterozygous carriers | 100 ± 15* | 373 ± 28 | 0.35 ± 0.11* | 13.7 ± 4.3*** |
| ASSD heterozygous carriers | 108 ± 36 | 282 ± 46 | 0.22 ± 0.03*** | 14.3 ± 8.4** |
Differences from control values are indicated; *, P < 0.05; **, P < 0.01; ***, P < 0.001. Differences from symptomatic OTC females: †, P < 0.01.
Reflects urea synthesis from therapeutic arginine or citrulline. When this is taken into account endogenous urea synthesis was −0.7 ± 16 μmol/(kg⋅h).
Reflects the effect of Ucephan on glutamine flux.
