Abstract
The anaphase promoting complex or cyclosome is the ubiquitin-ligase that targets destruction box-containing proteins for proteolysis during the cell cycle. Anaphase promoting complex or cyclosome and its activator (the fizzy and fizzy-related) proteins work together with ubiquitin-conjugating enzymes (UBCs) (E2s). One class of E2s (called E2-C) seems specifically involved in cyclin B1 degradation. Although it has recently been shown that mammalian E2-C is regulated at the protein level during the cell cycle, not much is known concerning the expression of these genes. Arabidopsis encodes two genes belonging to the E2-C gene family (called UBC19 and UBC20). We found that UBC19 is able to complement fission yeast (Schizosaccharomyces pombe) UbcP4-140 mutant, indicating that the plant protein can functionally replace its yeast ortholog for protein degradation during mitosis. In situ hybridization experiments were performed to study the expression of the E2-C genes in various tissues of plants. Their transcripts were always, but not exclusively, found in tissues active for cell division. Thus, the UBC19/20 E2s may have a key function during cell cycle, but may also be involved in ubiquitylation reactions occurring during differentiation and/or in differentiated cells. Finally, we showed that a translational fusion protein between UBC19 and green fluorescent protein localized both in the cytosol and the nucleus in stable transformed tobacco (Nicotiana tabacum cv Bright Yellow 2) cells.
Transition from one phase of the cell cycle to another is accomplished through changes of activity of key regulatory proteins. The correct progress through the cell cycle is thus under the control of successive events where protein activation alternates with protein degradation mediated by the ubiquitin-dependent proteolytic pathway. Degradation via this pathway is a two-step process: The protein is first tagged by the covalent attachment of polyubiquitin chain(s) and then degraded by the multicatalytic protease complex called the 26S proteasome (for reviews, see Hershko and Ciechanover, 1998; Ciechanover et al., 2000). Conjugation of ubiquitin to the protein involves a cascade of three enzymes: E1, E2, and E3. The E1 (ubiquitin-activating) enzyme forms a high-energy bond with ubiquitin, which is then transesterified to an ubiquitin-conjugating enzyme (UBC) (E2). The transfer of ubiquitin to the target protein substrate usually requires ubiquitin-ligase activity (E3). The specificity in targeting a protein for ubiquitylation resides primarily in cognate pairs of E2 and E3 enzymes.
At the onset and during anaphase, several key proteins are degraded; among them, mitotic cyclins (for review, see Murray, 1995), the anaphase inhibitors such as Brewer's yeast (Saccharomyces cerevisiae) Pds1p (Cohen-Fix et al., 1996) and fission yeast (Schizosaccharomyces pombe) Cut2p (Funabiki et al., 1996), the mitotic spindle associated Ase1p protein (Juang et al., 1997), the DNA replication inhibitor called geminin (McGarry and Kirschner, 1998), and the chromokinesin Xkid protein involved in chromosome alignment during metaphase (Funabiki and Murray, 2000). All of these substrates carry a nine-amino acid sequence called the destruction box (Dbox) motif. Another degradation signal, called the KEN-box, has also been recently identified (Pfleger and Kirschner, 2000). The E3 activity involved in this process is located on a large complex called APC/C or cyclosome, which is regulated by phosphorylation and also requires activator proteins called Cdc20/Fizzy and Cdh1/Fizzy-related (for review, see Morgan, 1999; Page and Hieter, 1999; Peters, 1999; Zachariae and Nasmyth, 1999). Although the mechanism of the Dbox or KEN-box recognition via the activator proteins has been solved very recently (for review, see Vodermaier, 2001), the identity of the E2 and its function during APC-dependent ubiquitylation is still poorly understood.
One E2 responsible for ubiquitylation of cyclin B, has been enzymatically purified and identified as E2-C in clam egg extract (Hershko et al., 1994; Sudakin et al., 1995; Aristarkhov et al., 1996) and as UBC-x in Xenopus sp. egg extracts (King et al., 1995; Yu et al., 1996). A fission yeast gene called UbcP4, which is involved in Cdc13 cyclin degradation and is essential for entry into anaphase, has also been identified (Osaka et al., 1997). Finally, the human ortholog of E2-C, named UbcH10, has been characterized (Townsley et al., 1997), and the overexpression of a dominant-negative form of this protein in transfected mammalian cells arrests the cells in mitosis. All of these proteins belong to a class of E2 constituted by the conserved core domain containing the catalytic Cys and an N-terminal extension whose function is still unclear. In budding yeast, the E2 enzyme most similar in sequence to E2-C, Ubc11p, does not carry the N-terminal extension domain and is not essential for yeast cell viability (Townsley and Ruderman, 1998). The genome sequence of C. elegans surprisingly did not reveal a gene structurally related to E2-C (Jones et al., 2001), indicating that C. elegans and budding yeast use one or other E2s for APC-mediated proteolysis. Yeast Ubc4p (another E2 member) supports cyclin Clb2p ubiquitylation in vitro, in the presence of the E1 enzyme and the APC ringH2 finger protein Apc11 (Leverson et al., 2000). Xenopus sp. and human orthologs of Ubc4p work also in concert with purified APC for the ubiquitylation of Dbox-containing proteins in vitro (King et al., 1995; Yu et al., 1996; Charles et al., 1998). Only human Ubc4 unexpectedly worked in in vitro ubiquitylation reactions with Apc11, but not the E2 UBC-x (Gmachl et al., 2000). Thus, although two classes of E2 (e.g. E2-C/UBC-x and UBC4) seem to be involved in the Dbox pathway, it is not yet clear how the E2s mediate APC-dependent protein ubiquitylation in vivo.
Furthermore, not much is known about the regulation of E2s expression during the cell cycle and in differentiated cells. It was shown that mammalian E2-C gene expression was up-regulated during oncogenic transformation (Arvand et al., 1998) and down-regulated in aging human fibroblasts, like several other genes involved in G2/M transition of the cell cycle (Ly et al., 2000). The work of Yamanaka et al. (2000) curiously suggested that mammalian E2-C is not regulated at the transcriptional but rather at the posttranslational level, because the protein is itself a substrate of APC/C dependent proteolysis.
Here, we describe the molecular characterization of two Arabidopsis E2s, called UBC19 and UBC20, structurally related to the UbcP4/E2-C/UBCx/UbcH10 protein family. We demonstrated the ability of the plant UBC19 to functionally complement the fission yeast UbcP4-140 mutant and investigated UBC19/20 expression patterns in various plant organs and tissues.
RESULTS
Arabidopsis Encodes Two Ubiquitin-Conjugating Enzymes Structurally Related to the UbcP4/E2-C/UBCx/UbcH10 Class of E2s
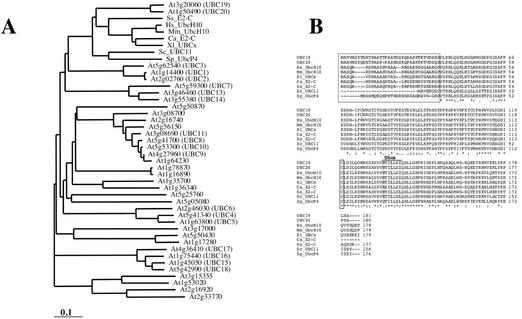
The sequences of the clam E2-C (Aristarkhov et al., 1996) and Xenopus sp. UBC-x (Yu et al., 1996) were used for Arabidopsis database searches and led to the identification of two different genes called UBC19 and UBC20, located on Arabidopsis chromosome 3 (At3g20060) and chromosome 1 (At1g50490), respectively. Both genes are expressed because their corresponding expressed sequence tags can be found in the currently available databases. The sequence of the longest expressed sequence tag for UBC19 was determined, and it encodes an 181 amino acids open reading frame which is complete at its N-terminal end as indicated by an in-frame stop codon upstream of the initiation codon. The protein sequence of UBC20 exhibits greater than 90% sequence identity to UBC19. A phylogenetic tree including all of the 37 E2s from Arabidopsis (Bachmair et al., 2001), indicated that UBC19/20 are more closely related to the UbcP4/E2-C/UBCx/UbcH10 E2s than any other Arabidopsis E2 (Fig. 1A). Bootstrap values derived from the neighbor-joining method (Saitou and Nei, 1987) were all above 55% inside of each clade (data not shown), further supporting the evolutionary relationship between the different E2 subfamilies. Both Arabidopsis UBC19 and UBC20 amino acid sequences were aligned with human, mouse, Goldfish, Xenopus sp., clam, and fission yeast E2-C orthologs and also Brewer's yeast UBC11 (Fig. 1B). The Arabidopsis E2 sequences contain the short N-terminal extension that distinguishes this subgroup from other E2s, with the exception of yeast UBC11. The plant proteins carry even the longest N-terminal extensions (36 amino acids for UBC19, compared with the 30 amino acid extensions in mammals, Xenopus sp., and clam).
Figure 1.
Phylogenetic tree and sequence alignments of the E2-C proteins. A, Phylogenetic tree performed with the Clustal program including all Arabidopsis E2s and the E2s from other organisms belonging to the E2-C class. Branch lengths are proportional to phylogenic distances. B, Alignment of the Arabidopsis UBC19 and UBC20 amino acid sequences with those of human UbcH10, mouse UbcH10, Xenopus sp. UBCx, goldfish E2-C, clam E2-C, Brewer's yeast Ubc11p, and fission yeast UbcP4. Multiple sequence alignment was performed with ClustalW (1.81) program. The N-terminal extension domains and the active Cys residues are boxed. The localization of the Dbox sequences is indicated. Asterisks and dots indicate identical and conserved amino acids, respectively. UBC19 and UBC20 nucleotide and amino acid sequences have been submitted to the EMBL/GenBank sequence libraries under accession numbers AY127573 and AY127574, respectively.
The crystal structure of clam E2-C has recently been solved (Jiang and Basavappa, 1999). The authors showed that most of the residues that are specifically conserved into this E2 class of enzymes contribute rather to the protein surface, suggesting that they are at the molecular interface for protein interaction and specificity. Most of these residues are conserved in the plant E2 protein sequences (residues V31, Q36, M44, F53, D55, T65, D108, T130, G140, L148, N158, K163, and Y170; the identity and number of the residues is based on clam sequence).
UBC19 Protein Forms a Thiol-Ester Bond with Ubiquitin in Vitro
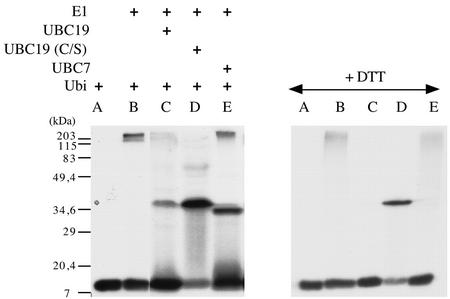
In E2s, the active site Cys forms a thiol-ester linkage with the C-terminal carboxyl of ubiquitin. The coding region of Arabidopsis UBC19 cDNA was cloned in the pET11d-inducible expression vector, and the enzyme was overexpressed in Escherichia coli. A mutant version of the protein, in which the catalytic Cys was replaced by a Ser residue, was also produced in E coli. Bacterial protein extracts expressing either the wild type or the mutant protein were assayed in vitro for ester formation of the recombinant polypeptides with radiolabeled-ubiquitin in the presence of Arabidopsis ubiquitin-activating enzyme E1 (UBA1; Hatfield et al., 1997) and ATP (Fig. 2). As a control, we used purified Arabidopsis UBC7 protein. In the presence of the bacterial extracts, a radiolabeled protein complex with a size of around 36 kD could be detected (Fig. 2, lanes C and D). As expected, the complexes formed between the native UBC19 (or UBC7) and ubiquitin were sensitive to boiling under reducing conditions, indicating that the proteins are linked via a thiol-ester bond (Fig. 2, +DTT, lanes C and E). The protein complex with the mutant protein UBC19 (C/S) was resistant to boiling in the presence of DTT, most likely because of the formation of an oxygen-ester (lane D). Finally a mutant version of the protein, in which the catalytic Cys was replaced by an Ala residue, UBC19 (C/A), was unable to form a complex with ubiquitin (data not shown).
Figure 2.
Thioester complex formation between ubiquitin and the UBC19 protein. Assay reactions contained ATP, 32P-labeled ubiquitin overexpressed in E. coli, purified Arabidopsis E1 enzyme UBA1, and crude extracts from bacteria expressing UBC19 (lane C), UBC19 (C/S; lane D), or UBC7 (lane E) protein. Reactions were analyzed by SDS-PAGE under nonreducing or reducing (+dithiothreitol [DTT]) conditions, and radiolabeled proteins were visualized by autoradiography. Control reactions containing labeled ubiquitin incubated with non-transformed bacterial extracts with (lane B) or without (lane A) the E1 enzyme are shown. Extracts from bacteria expressing UBC19 incubated with 32P-labeled ubiquitin but without the E1 enzyme were unable to form the thiolester complex (data not shown).
UBC19 Complements Fission Yeast Mutant UbcP4-140
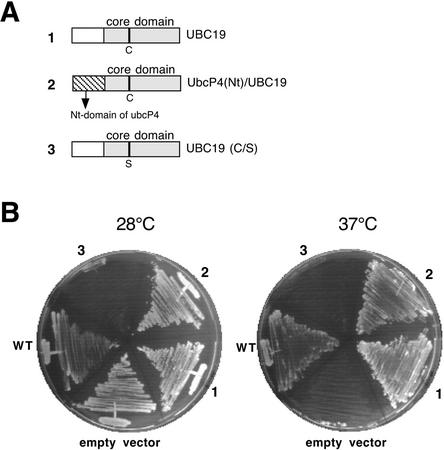
Disruption of the fission yeast gene UbcP4 produces both a G2/M and a metaphase/anaphase cell cycle arrest (Osaka et al., 1997; Mitsuzawa et al., 2001). We tested whether the plant protein is able to rescue the fission yeast ts mutant UbcP4-140 (Mitsuzawa et al., 2001). For the complementation experiments, different constructs were designed (see Fig. 3A): the full-length UBC19, a dominant negative version of UBC19 (C/S) and a chimeric gene, in which the N-terminal extension domain of UBC19 was replaced by the N-terminal extension domain of yeast UbcP4. The different constructs were put under the control of the thiamine-repressible promoter pnmt1 of the pREP1 expression vector and transformed into UbcP4-140 cells. At nonpermissive temperature and in the absence of thiamine, both the plant E2 and the chimeric E2 allowed the growth of UbcP4-140 mutant (Fig. 3B), whereas another Arabidopsis E2 UBC9 (At4g27960) belonging to a different E2 subfamily was unable to complement the mutant strain (data not shown). Interestingly, the chimeric protein [UbcP4(Nt)/UBC19] was more efficient for the growth rescue of the yeast mutant strain than the wild-type plant E2 (data not shown), suggesting that the N-terminal extension may carry important structural information involved in the E2 function. As expected, UBC19 (C/S) worked as a dominant negative mutant protein because it inhibited the growth of UbcP4-140 mutant strain already at permissive temperature. If this strain was grown at permissive temperature but in the presence of thiamine, which represses UBC19 (C/S) expression, no growth defect was detected (data not shown).
Figure 3.
Functional complementation of fission yeast temperature sensitive UbcP4-140 strain. A, Schematic representation of the constructs used for yeast complementation. All constructs were put into the pREP1 vector. Open and hatched boxes represent the N-terminal extension domains of UBC19 and Sp_UbcP4, respectively. The gray boxes represent UBC19 core domain. The catalytic Cys or the Ser (in the dominant negative version of the protein) residues are also represented. B, The yeast mutant strain was transformed with either an empty pREP1 vector or with the pRep1 vector expressing wild-type UBC19 (1) or chimeric UbcP4(Nt)/UBC19 (2) or the dominant negative UBC19 (C/S; 3). The wild-type yeast strain (WT) and the transformed UbcP4-140 strains were grown either at permissive (28°C) or restrictive (37°C) temperature.
Southern-Blot Analysis
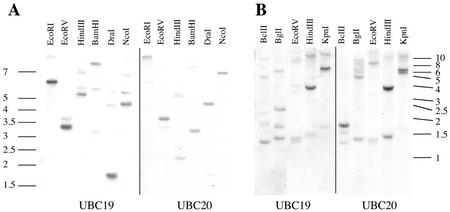
To investigate the expression patterns of both genes by RNA gel and in situ hybridization, we tested first the specificity and cross-reactivity of the cDNA probes. Genomic DNA from Arabidopsis and the closely related radish (Raphanus sativus cv Radis rubine scarlate) plant was digested by different restriction enzymes and hybridized with both UBC19 and UBC20 full-length cDNA probes (Fig. 4). In both Arabidopsis and radish, only weak cross-reactivity of the probes was detected. Furthermore the UBC19/20 cDNA probes did not cross-react with other E2 genes, like those belonging to the Ubc4/5 gene family (data not shown).
Figure 4.
Genomic Southern-blot analysis. Five micrograms of Arabidopsis (A) or 7 μg of radish (B) DNA was digested with the indicated restriction enzymes before electrophoresis in an agarose gel and subsequent transfer to a nylon membrane. The same membranes were successively probed with UBC19 (left panels) and UBC20 (right panels) cDNA probes. Size markers are indicated in kilobase pairs.
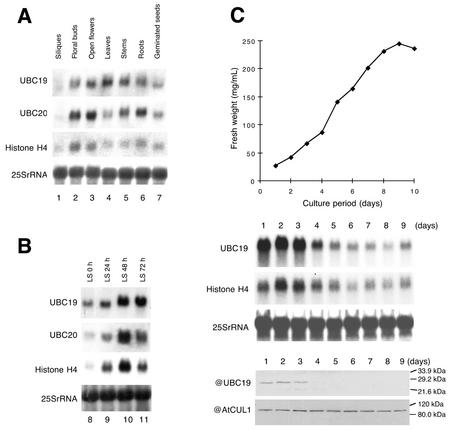
UBC19 and UBC20 mRNAs Accumulate in All Arabidopsis Organs and to a Higher Extent in Dividing Cells
Both UBC19 and UBC20 mRNAs were found to accumulate in various Arabidopsis organs and in germinating seeds (Fig. 5A). Expression of both genes was similar but not identical. Both UBC19 and UBC20 mRNAs accumulate in floral buds, open flowers, stems, and roots and at low levels in siliques. Nevertheless, UBC19 is expressed at higher level in mature leaves than UBC20. We also tested UBC19 gene expression under different growth conditions including heat shock, dark to light transition and drug treatments inducing a stress response (like the proteasome inhibitor MG132 or geldanamycin); none of these experimental conditions changed its expression level (data not shown).
Figure 5.
UB19 and UBC20 mRNA accumulation in various Arabidopsis organs, during re-initiation of mitotic activity in leaf strip cultures and in a cell-suspension culture. A, Total RNA was extracted from silique (lane 1), floral buds (lane 2), open flowers (lane 3), leaves (lane 4), stems (lane 5), roots (lane 6), and 3-d-old germinating seeds (lane 7); B, total RNA was extracted from leaf strips (LS) incubated in a medium suitable for cell division for 0 h (lane 8), 24 h (lane 9), 48 h (lane 10), and 72 h (lane 11). The blots were probed successively with UBC19, UBC20, histone H4, and 25S rRNA probes. C, Growth curve of the cell-suspension culture determined by the fresh weight (top right panel). Northern analysis from total RNA extracted at different times during the culture (middle right panel). The RNA blot was probed with UBC19, histone H4, and 25S rRNA probes. UBC19 protein accumulation at different times during the culture (lower right panel). The western blot was probed successively with anti-UBC19 and anti-AtCUL1 polyclonal antibodies.
To study the E2 gene expression during re-initiation of mitotic activity in Arabidopsis, we used an experimental system (Genschik et al., 1994) in which differentiated leaf cells are stimulated to re-enter the cell cycle by wounding and auxin stimulation. Both UBC19 and UBC20 mRNAs accumulate in the leaf strips culture at the highest levels at 48 to 72 h (Fig. 5B), a time when the first cell divisions are observed (data not shown). Histone H4 mRNAs accumulate at the highest level earlier at 24 to 48 h, when most of the cells in the culture are in S phase (24 to 48 h of culture corresponding to the highest 3H-dTTP incorporation; data not shown).
We also followed UBC19 mRNA and protein levels during Arabidopsis cell-suspension culture (Fig. 5C). Cells rapidly entered the logarithmic growth phase and reached the stationary stage after 7 d. Both histone H4 and UBC19 mRNAs reached their highest level of accumulation from d 1 to 3 (corresponding to the time where the highest number of cell division can be observed; data not shown). A polyclonal antibody was raised in rabbits against the 20-amino acid N-terminal peptide of UBC19 and used in western-blot analysis. The UBC19 protein was only detectable during the first days of culture corresponding to the highest level of its transcript accumulation. As a control, we used an antibody raised against AtCUL1, whose gene is constitutively expressed in Arabidopsis cell suspension culture (Shen et al., 2002).
UBC19 and Ubc20 Genes Are Expressed in Various Arabidopsis and Radish Tissues
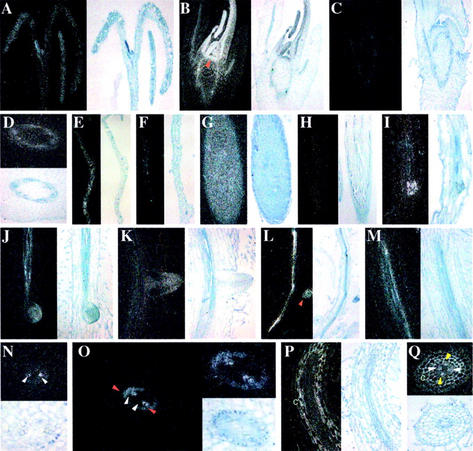
To analyze more precisely and identify the tissues in which the plant E2s are expressed, we performed in situ hybridization using [35S] UTP-labeled UBC19 and UBC20 anti-sense or sense probes on different sections of Arabidopsis and of its very closely related radish species (Fig. 6). Radish organs are larger than Arabidopsis and were therefore used to obtain more accurate and detailed mRNA localization. In general, both UBC19 and UBC20 probes revealed almost identical expression patterns, except in root tissues (see below). Thus, in situ hybridization with the UBC20 probe is only shown when different from UBC19.
Figure 6.
In situ localization of UBC19 and UBC20 mRNAs in various Arabidopsis and radish organs. Hybridization signals visualized as white grains in dark field optics are shown on left panels, whereas bright field images showing tissue morphology are shown on right panels. In situ hybridization with control sense probes for both E2s were systematically performed with sections of different organs analyzed and gave only very weak background signals (only shown for shoot and root apexes). A, Longitudinal sections through Arabidopsis seedlings 5 d after germination shows UBC19 expression in shoot meristem and young leaves. B, Longitudinal section through radish shoot apex shows expression of UBC19 in the shoot apical meristems and young leaves. Arrowhead points to the shoot apical meristem. C, Longitudinal section through a shoot apex of radish hybridized with UBC19 sense probe. D, Transverse section of a radish stem shows expression of UBC19 in the vascular tissue. E and F, Longitudinal sections through differentiated Arabidopsis leaves hybridized with UBC19 (E) and UBC20 (F) anti-sense probes. G, Longitudinal section through radish root apex illustrating homogeneous expression of UBC19 in root meristems. H, Longitudinal section through radish root apex with UBC19 sense probe. I, Longitudinal section through an Arabidopsis root showing UBC19 expression in emerging lateral root meristem. J and K, Longitudinal sections through radish roots showing UBC19 expression in emerging (J) and in emerged (K) lateral root meristems. L and M, Longitudinal sections of Arabidopsis (L) and radish (M) roots showing UBC19 transcript localization in the vascular cylinder. N, Transverse section of radish roots showing high expression of UBC19 in protoxylem elements (white arrowheads). O, Transverse sections of differentiating radish root further away from the root tip than sections shown in N illustrating UBC19 expression in pericycle cells at the xylem poles (red arrowheads) and xylem elements (white arrowheads). P and Q, Longitudinal (P) and transverse (Q) sections of radish roots showing UBC20 expression in protoxylem elements (white arrowheads). Expression in phloem (yellow arrowheads) and cortex tissue (c) is only observed with UBC20 anti-sense probe.
UBC19/20 genes are expressed in the shoot apical meristem, leaf primordial, and young leaves (Fig. 6, A and B). Sense probes never allowed detection of a signal either with Arabidopsis or radish sections, as illustrated with the UBC19 sense probe (Fig. 6C). In stems, transcript localization of both E2s is seen in the vascular tissue (shown for UBC19 in Fig. 6D). According to the RNA gel analysis (see Fig. 5A), UBC19 is expressed at higher levels in mature leaves than UBC20 (Fig. 6, E and F). UBC19/20 genes are also expressed in the root meristem and in emerging lateral roots (Fig. 6, G and I–L). Hybridization performed with sense probes gave only weak background signals, as illustrated in Figure 6H.
However, whereas UBC19 is only expressed in the vascular cylinder (Fig. 6, J, L, and M), mainly in protoxylem elements (Fig. 6, M and N), UBC20 probe allowed also the detection of a signal in differentiated root cortex, xylem, and phloem tissues (Fig. 6, P and Q). Furthermore both genes were expressed in pericycle cells in maturing roots at the xylem poles, where potentially lateral roots emerge (Fig. 6O; data not shown). Because the two E2 probes exhibit weak cross-hybridization (Fig. 4) and because the number of radish genes belonging to this E2 family is unknown, we cannot exclude that this expression pattern results from the superposition of the expression of several, still yet unknown, radish UBC19/20 homologs. To determine to what extent this E2 gene family is subjected to differential tissue-specific expression, additional experiments are required. Differential tissue-specific expression has already been reported for the Arabidopsis UBC4-6 gene family (Thoma et al., 1996), using promoter β-glucuronidase (GUS) fusion studies.
Thus, the in situ hybridization experiments revealed that in all tissues with cell division activities, UBC19/20 expression could be detected: like the shoot and root meristems, expanding vascular tissues, and leaf primordial. However, the expression of these genes is not exclusively correlated with cell proliferation, because we found also expression in differentiated cells (in mature leaves and in root cortex).
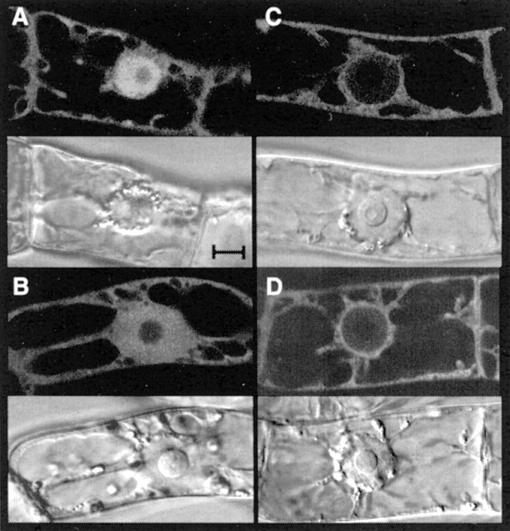
UBC19/Green Fluorescent Protein (GFP) Fusion Protein Is Both Cytoplasmic and Nuclear
For subcellular localization studies, UBC19 was translationally fused to the GFP proteins, and stable transgenic tobacco (Nicotiana tabacum cv Bright Yellow 2 [BY2]) cells, expressing the fusion protein under the control of the Dex-inducible promoter (Aoyama and Chua, 1997), were established. The UBC19/GFP fusion protein, like GFP alone, was found both in the cytoplasm and the nucleus (Fig. 7, A and B). The size of the fusion protein is around 46 kD, which is close to the selective barrier (20–40 kD) of passive protein diffusion (for review, see Görlich and Kutay, 1999). To investigate whether UBC19 carries the capacity to translocate a reporter protein in the nucleus, we engineered a construct in which UBC19 was fused in frame to both GFP and GUS, increasing the size of the reporter protein to more than 100 kD. Nevertheless, this was not the case, and the UBC19/GFP/GUS fusion protein, like the GFP/GUS control protein, localized mainly to the cytoplasm (Fig. 7, C and D), indicating that UBC19 enters the nucleus only by passive diffusion.
Figure 7.
Subcellular localization of UBC19 using GFP as life marker in tobacco BY2 cells. Each fluorescent focal plane (top panels) is shown with its corresponding transmitted light reference image viewed by differential interference contrast (bottom panels). Localization of GFP alone (A), UBC19-GFP (B), GUS-GFP (C), and UBC19-GUS-GFP (D) in interphase BY2 cells after 24 h of Dex induction. Bar = 10 μm.
DISCUSSION
It is now clear that the Dbox pathway is regulated at multiple levels during the cell cycle (for reviews, see Morgan, 1999; Page and Hieter, 1999; Zachariae and Nasmyth, 1999). Thus, the core APC/C ubiquitin ligase is itself regulated during the cell cycle by phosphorylation of several of its subunits. Furthermore, it has been shown that APC/C activity requires activator proteins, called Cdc20/Fizzy and Cdh1/Fizzy-related. The first class of proteins is regulated both at the transcriptional and posttranscriptional (e.g. proteolysis) levels, whereas the later is mainly regulated by phosphorylation. Nevertheless, not much is known concerning the contribution of the E2 enzymes to the APC/C activity. Two classes of E2s (E2-C/Ubc-x and Ubc4), capable of contributing to APC activities have been reported (see the introduction). Although both classes of E2s were able to work with purified APC/C in vitro, in vivo functional data are only available for the E2-C class of proteins. In fission yeast, the mutation of the gene (called UbcP4) produced cell cycle arrests (at G2/M and at metaphase-to-anaphase transition) and inhibited mitotic cyclin Cdc13 degradation (Osaka et al., 1997). In animal cells, overexpression in COS cells of a dominant negative version of the human E2-C protein (UbcH10) inhibited cyclin A and B1 degradation, whereas a dominant negative clam E2-C protein inhibited the onset of anaphase in frog embryonic cells (Townsley et al., 1997). Specificity of the E2 involved in mitotic events was assayed by microinjecting PtK1 cells with different dominant negative E2s (Bastians et al., 1999). These experiments clearly demonstrated that only dominant negative human UbcH10 and not UbcH5b, caused a dramatic delay in anaphase onset, suggesting that E2-C is selectively required for anaphase inhibitor degradation.
Nevertheless, how these E2s are regulated during the cell cycle and in differentiated cells is poorly understood. Whereas the fission yeast UbcP4 protein seems to accumulate constitutively during the cell cycle (Osaka et al., 1997), the mammalian E2-C is mainly regulated at the posttranslational level by APC/C-dependent proteolysis of the protein (Yamanaka et al., 2000). RNA gel-blot analysis revealed that the goldfish E2-C mRNAs accumulate strongly in ovaries similarly to cyclin B mRNAs (Tokumoto et al., 1999).
Arabidopsis, whose genome sequencing has been achieved (The Arabidopsis Genome Initiative, 2000), encodes thirty-seven potential different E2 genes (for review, see Bachmair et al., 2001). The E2 class, to which budding yeast Ubc4 belongs, is represented in Arabidopsis by a multigenic family of eight members, whereas for the E2-C class of E2s, only two genes could be identified (UBC19 and UBC20). We demonstrated that at least one of the plant genes could replace the fission yeast UbcP4 gene for its cell cycle functions. Thus, the plant proteins are both structurally and functionally related to the E2-C class of enzymes.
Expression analyses, in Arabidopsis and radish, were performed both by RNA gels and in situ hybridization. Both UBC19 and UBC20 genes are more strongly expressed in tissues active for cell division, similarly to cyclin B1 (Ferreira et al., 1994; Segers et al., 1996). Interestingly, Arabidopsis UBC19/20 promoter sequences contain a common cis-acting element, called the M-phase-specific activator element (data not shown), which is necessary and sufficient for G2/M specific gene expression (for review, see Ito, 2000). Nevertheless, whether UBC19/20 expression occurs at a particular stage of the cell cycle remains to be demonstrated. Furthermore, it is worth to note that a strong expression in Arabidopsis meristematic regions has also been reported for E1 and other E2 genes (Thoma et al., 1996; Hatfield et al., 1997), suggesting a particular need of ubiquitylation during cell proliferation.
Although UBC19/20 expression was always detected in tissues where cell division occurs, we also found some expression in differentiated tissues. Thus, a higher level UBC19 mRNAs accumulated in mature leaves, whereas expression of UBC19/20 radish homologs was detected in differentiating and mature cortex. In animal cells, it was shown that Dbox pathway is active in in vitro differentiated myoblast cells (Brandeis and Hunt, 1996) and in terminally differentiated neurons (Gieffers et al., 1999). By using a chimeric fusion protein (the N-terminal domain of cyclin A3 fused to the GUS reporter protein), we found that the Dbox pathway is active in several differentiated tobacco tissues (A. Derevier, M.C. Criqui, and P. Genschik, unpublished data). Substrates for APC/C-dependent ubiquitylation in differentiated cells are unknown. Nevertheless, this raises the interesting possibility that APC/C dependent protein degradation occurs outside of the cell cycle, as already suggested by J.-M. Peters and collaborators (Gieffers et al., 1999) and that UBC19/20 and their orthologs may contribute to this proteolytic pathway.
MATERIALS AND METHODS
Unless stated otherwise, all procedures for manipulating DNA and RNA were carried out according to Sambrook et al. (1989) and Ausubel et al. (1994). Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Chemicals
Dexamethasone (Sigma-Aldrich, St. Louis) was dissolved in ethanol and stocked at a concentration of 30 mm.
Construction of Plasmids
To construct the dominant negative pSK-UBC19(C/S), we mutated the Cys residue in the UBC domain, involved in thiol-ester formation with ubiquitin, by PCR, using oligonucleotide (5′-CAATATTAGCTTGGACATTCTTC-3′) and oligonucleotide (5′-CCATAGAGATCCACATTGG-3′), respectively. The three constructs for yeast complementation assays were cloned as SalI-BamHI fragments into the pREP expression vector (Maundrell, 1993). These restriction sites were introduced for UBC19 and UBC19 (C/S) by PCR amplification using oligonucleotide 5′-AGGCGAGTCGACATGGCGACGGTTAATGGGTAC-3′ and oligonucleotide 5′-CAGTGATTTTGGATCCTCATGCGTTTAAAGGCT-3′. The chimeric UbcP4/UBC19 fragment has been generated with the Exsite PCR site-directed mutagenesis kit (Stratagene, La Jolla, CA) with oligonucleotide 5′-AATCTAAAAACTCTTCTTCTGCTGGCATGGCTGTTGACGGTCATAGCGTTCTAAAAAGGCTGCAATCTGAAC-3′ and oligonucleotide 5′-AGTATGAGGGTTCTGGTTTTGCATATCAGAATCCATCCTTTTTCGCCTCTTCTAATTG-3′. pTA-GFP construct is described in Criqui et al. (2000). The GFP sequence used in all experiments is the ultra-bright version of the [Leu 64 > Phe] and [Ser 65 > Thr] (see Menand et al., 1998). A XhoI-NcoI fragment of UBC19 obtained by PCR amplification using oligonucleotide 5′-CATTAACTCGAGCCATGGCGACGGTTAATGGGTACACAG-3′ and oligonucleotide 5′-AATTAACCATGGATGCGTTTAAAGGCTTG-3′ was introduced in frame at the N terminus of GFP and resulted in plasmid pSK-UBC19-GFP. The XhoI-SpeI fragment was than subcloned into the dexamethasone-inducible vector pTA7002 (Aoyama and Chua, 1997) resulting in plasmid pTA-UBC19-GFP. In the pTA-GUS-GFP construct, the GUS coding sequence was introduced in frame with the GFP sequence after PCR amplification with oligonucleotide 5′-GATCATCTCGAGACCATGGTACGTCCTGTAGAAACCCCAA-3′ and oligonucleotide 5′-ACTCATGGATCCTTGTTTGCCTCCCTGCTGCGGTTT-3′. Finally, the pTA-UBC19-GUS-GFP construct resulted from subcloning the UBC19 coding sequence from the pSK-UBC19-GFP construct into pTA-GUS-GFP vector. The PCR-amplified fragments in all cloning procedures were always sequenced on both strands.
Plant Material
The Arabidopsis plants used for the RNA gels were of the Wassilewskija ecotype. To check UBC19 expression during the dark to light transition, Arabidopsis plants have been grown in vitro in complete darkness for 12 d, and then were transferred to standard light condition and collected after 1, 3, and 8 h. A gene encoding a photosystem II component was used as a control. For heat shock, 14-d-old Arabidopsis plants grown in vitro under standard conditions were collected and incubated in sterile water either at 38°C for the heat shock or at 22°C for the control. After 90 min of treatment, plants were incubated in sterile water at 22°C and collected 1 h later for RNA preparation. Control hybridization experiments with HSP70 and HSP105 probes, indicated a strong induction of HSP genes under our experimental conditions.
Clonal transgenic tobacco (Nicotiana tabacum) BY2 cell cultures for pTA-GFP, pTA-UBC19/GFP, pTA-GUS/GFP, and pTA-UBC19/GUS/GFP constructs were established. The Agrobacterium tumefaciens-mediated transformation protocol of the BY2 cells is described by Genschik et al. (1998). To produce the accumulation of the GFP fusion proteins into the transgenic cell lines, 5 μm dexamethasone was added to the culture medium. The handling of the BY2 cell culture were performed according to Nagata et al. (1992).
RNA and DNA Gel Blotting
RNA gels were realized with 20 μg of total RNA per lane. The RNA extraction and RNA gel-blotting procedures are described by Criqui et al. (2000). The UBC19 and UBC20 cDNA probes correspond to the complete cDNAs clones. The histone H4 probe corresponds to the 196-bp restriction fragment AccI-DdeI of the coding region of the gene H4A748 (Chaboute et al., 1987). The integrity and the amount of RNA applied to each lane were verified by ethidium bromide staining and control hybridizations using an Arabidopsis 25S rRNA probe (GenBank accession no. T44938).
The genomic DNA was extracted according to (Qiagen USA, Valencia, CA). The UBC19 and UBC20 cDNA probes correspond to the complete cDNAs clones. The DNA gel blots were hybridized overnight at 42°C in 5× SSPE, 50% (v/v) formamide, 10% (w/v) dextran sulfate, 1% (w/v) SDS, and 50 μg mL−1 denatured salmon sperm DNA. The blots were subsequently washed in 2× SSC and 0.1% (v/v) SDS for 30 min at 42°C and in 0.2× SSC and 0.1% (v/v) SDS for 30 min at 42°C.
In Situ Hybridizations
Virtually all steps of the in situ procedure were performed essentially as described by de Almeida Engler et al. (2001). Radish (Raphanus sativus cv Radis rubine scarlate) and Arabidopsis (ecotype Columbia) seeds were germinated in K1 medium (Valvekens et al., 1988), and plant material was harvested for fixation. All samples were fixed in 2.5% (v/v) glutaraldehyde, dehydrated, and paraffin embedded. Sections of 10 μm thick were fixed to 3-aminopropyltriethoxy-silane-coated slides and used during the in situ hybridization procedure. UBC19 and UBC20 probes were synthesized from PCR products flanked by T3 and T7 promoters and 20 × 106 cpm slide−1 were applied (10 ng slide−1). Exposure times varied and images were taken by using a digital Axiocam (Zeiss, Welwyn Garden City, UK) under standard dark and bright field optics.
Overexpression of the E2 Enzymes and Ubiquitin-E2 Thiol-Ester Linkage
NcoI and BamHI sites were introduced 5′ and 3′, respectively, of pSK-UBC19 and pSK-UBC19 (C/S) by PCR-based site-directed mutagenesis. The PCR reaction was performed using oligonucleotide 5 (5′-GCGAAAAACCATGGCGACGGTTAATG-3′) and oligonucleotide 2 (5′-ATTTTTGAGGATCCTCATGCGTTTAAAGGCTAG-3′) as the upstream and downstream primers, respectively. The NcoI-BamHI fragments were cloned into the pET-11d vector (Novagen, Madison, WI) to produce recombinant proteins with an additional SHHHHHH sequence at the C terminus. Overexpression was performed in Escherichia coli strain BL21(DE3) pLysS. The recombinant His-tagged ubiquitin containing a cAMP-dependent protein kinase phosphorylation site (Tan et al., 1999) was kindly provided by Dr. Zhen-Qiang Pan and was expressed in E. coli strain BL21(DE3) pLysS. The protein remained soluble and could be purified in the native form under non-denaturing conditions using the nickel-nitrilotriacetic acid agarose-based affinity purification procedures according to the protocol provided by Qiagen USA. The purified protein was further applied to a Sephadex G-25 column, equilibrated, and eluted with 50 mm NaH2PO4 (pH8), 300 mm NaCl, and 10 mm phenylmethylsulfonyl fluoride.
One microgram of the ubiquitin fusion protein was radiolabeled using 5 units of cAMP-dependent protein kinase (Biolabs) and 10 μCi of [γ32P]ATP as described by Tan et al. (1999). After the reaction the kinase was heat inactivated. For thioester assays, reactions were carried out in 50 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 1 mm ATP, and 100 ng of 32P-labeled ubiquitin at 30°C for 40 min. Unless stated otherwise, 500 ng of the purified Arabidopsis E1 enzyme UBA1 (Hatfield et al., 1997) and 10 μg of bacterial crude protein extracts overproducing UBC19 or its mutant proteins were used in the reaction. The protein samples (in presence or absence of DTT) were subjected to electrophoresis in a 15% (w/v) SDS-polyacrylamide gel, and autoradiography was used to visualize radiolabeled bands.
Protein Extraction, Preparation of Antibodies, and Immunoblotting
The Arabidopsis cells were homogenized using pestle and mortar in extraction buffer (25 mm Tris-HCl, pH 7.5, 15 mm MgCl2, 15 mm EGTA, 150 mm NaCl, 0.1% [w/v] Tween 20, 1 mm DTT, and complete protease inhibitor cocktail mix [Roche Diagnostics, Indianapolis]) with PolyClar AT (Serva, Garden City Park, NY) and quartz and centrifuged at 20,000g. The protein content was determined by using the Bio-Rad protein assay kit.
Polyclonal antibodies were raised in rabbits against the N-terminal ATVNGYTGNTPAATTPAAT peptide of UBC19 protein to which an additional Cys residue at the N terminus was added for keyhole limpet hemocyanin coupling. Samples of 15 μg of proteins were separated by 15% (w/v) SDS-PAGE gels and transferred to Immobilon-P membrane (Millipore, Bedford, MA). The membranes were probed with the anti-UBC19 antibody diluted 1:3,000 (v/v). For protein loading control, we used a polyclonal antibody raised against Arabidopsis AtCUL1 protein (Shen et al., 2002). The antibody was used at a dilution of 1:2,500 (v/v). The immunoreactive proteins were detected using peroxidase-conjugated goat anti-rabbit antibodies (Dianova, Hamburg, Germany) and the ECL western-blot analysis system from Amersham Biosciences AB (Uppsala). Determination of the specificity of the antibodies was performed by competition experiments (data not shown). In this case, the antibody was incubated for 3 h at room temperature without or with 0.1 μm of antigen peptide before application to the membranes.
UbcP4-140 Yeast Strain Complementation
Fission yeast (Schizosaccharomyces pombe) UbcP4-140 ts mutant strain (h-ade6-M210 leu1-32 ura4-D18 ubcP4-140::ura4) established from strain HM530 (h-ade6-M210 leu1-32 ura4-D18) was kindly provided by Dr. Hiroaki Seino (Shizuoka, Japan; Mitsuzawa et al., 2001). For the manipulation of these strains, we used the conditions described by Guthrie and Fink (1991). Between three and six independent transformants per constructs were assayed in the complementation experiments that were repeated at least twice. The same results were obtained for the different experiments.
Fluorescence Imaging
Confocal images were obtained by a Zeiss LSM510 laser-scanning confocal microscope with argon laser excitation at 488 nm and through 505 to 550 emission filter-set and using a C-APOCHROMAT (63×1, 2-W Korr) water objective lens. The images are presented as single sections. Transmitted light reference images were taken using differential interference contrast (Nomarski) optics and argon laser illumination at 488 nm. Images were contrast enhanced using image-processing software (Photoshop, Adobe Systems Inc., Mountain View, CA).
ACKNOWLEDGMENTS
We thank Nam-Hai Chua for the inducible vector pTA7002; Zhen-Qiang Pan for pET-15b-His-HA-Ub plasmid; Hiroaki Seino for fission yeast ubcP4-140 mutant strain; Alain Girod for providing the purified Arabidopsis UBC7 and UBA1 enzymes; the Arabidopsis Biological Resource Center for providing the 25S RNA clone; Tobacco Science Research Laboratory, Japan Tobacco, for allowing us to use the TBY2 cell suspension; l'Université Louis Pasteur de Strasbourg, Centre National de la Recherche Scientifique, Association pour la Recherche sur le Cancer, La Ligue Nationale Contre le Cancer, and Région Alsace for founding the confocal microscope; Esther Lechner for technical help; Philippe Hammann for DNA sequencing; and Wen-Hui Shen for critical reading of the manuscript.
Footnotes
This work was supported by Action Concertée Incitative “Jeune Chercheur” from the French Ministry of Research.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011353.
LITERATURE CITED
- Aoyama T, Chua NH. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997;11:605–612. doi: 10.1046/j.1365-313x.1997.11030605.x. [DOI] [PubMed] [Google Scholar]
- The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Aristarkhov A, Eytan E, Moghe A, Admon A, Hershko A, Ruderman JV. E2-C a cyclin-selective ubiquitin carrier protein required for the destruction of mitotic cyclins. Proc Natl Acad Sci USA. 1996;93:4294–4299. doi: 10.1073/pnas.93.9.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvand A, Bastians H, Welford SM, Thompson AD, Ruderman JV, Denny CT. EWS/FLI1 up regulates mE2-C a cyclin-selective ubiquitin-conjugating enzyme involved in cyclin B destruction. Oncogene. 1998;17:2039–2045. doi: 10.1038/sj.onc.1202129. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: Greene Publishing; 1994. [Google Scholar]
- Bachmair A, Novatchkova M, Potuschak T, Eisenhaber F. Ubiquitylation in plants: a post-genomic look at a post-translational modification. Trends Plant Sci. 2001;6:463–470. doi: 10.1016/s1360-1385(01)02080-5. [DOI] [PubMed] [Google Scholar]
- Bastians H, Topper LM, Gorbsky GL, Ruderman JV. Cell cycle-regulated proteolysis of mitotic target proteins. Mol Biol Cell. 1999;10:3927–3941. doi: 10.1091/mbc.10.11.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis M, Hunt T. The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J. 1996;15:5280–5289. [PMC free article] [PubMed] [Google Scholar]
- Chaboute ME, Chaubet N, Philipps G, Ehling M, Gigot C. Genomic organization and nucleotide sequences of two histone H3 and two histone H4 genes of Arabidopsis thaliana. Plant Mol Biol. 1987;8:179–191. doi: 10.1007/BF00025329. [DOI] [PubMed] [Google Scholar]
- Charles JF, Jaspersen SL, Tinker-Kulberg RL, Hwang L, Szidon A, Morgan DO. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S cerevisiae. Curr Biol. 1998;8:497–507. doi: 10.1016/s0960-9822(98)70201-5. [DOI] [PubMed] [Google Scholar]
- Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. BioEssays. 2000;22:442–451. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O, Peters JM, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- Criqui M-C, Parmentier Y, Derevier A, Shen W-H, Dong A, Genschik P. Cell cycle-dependent proteolysis and ectopic overexpression of cyclin B1 in tobacco BY2 cells. Plant J. 2000;24:763–773. doi: 10.1111/j.1365-313x.2000.t01-1-.x. [DOI] [PubMed] [Google Scholar]
- de Almeida Engler J, De Groodt R, Van Montagu M, Engler G. In situ hybridization to mRNA of Arabidopsis tissue sections. Methods. 2001;23:325–334. doi: 10.1006/meth.2000.1144. [DOI] [PubMed] [Google Scholar]
- Ferreira PC, Hemerly AS, Engler JD, van Montagu M, Engler G, Inzé D. Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell. 1994;6:1763–1774. doi: 10.1105/tpc.6.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H, Murray AW. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell. 2000;102:411–424. doi: 10.1016/s0092-8674(00)00047-7. [DOI] [PubMed] [Google Scholar]
- Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- Genschik P, Criqui MC, Parmentier Y, Derevier A, Fleck J. Cell cycle-dependent proteolysis in plants: identification of the destruction box pathway and metaphase arrest produced by the proteasome inhibitor MG132. Plant Cell. 1998;10:2063–2076. doi: 10.1105/tpc.10.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschik P, Jamet E, Philipps G, Parmentier Y, Gigot C, Fleck J. Molecular characterization of a beta-type proteasome subunit from Arabidopsis thaliana co-expressed at a high level with an alpha-type proteasome subunit early in the cell cycle. Plant J. 1994;6:537–546. doi: 10.1046/j.1365-313x.1994.6040537.x. [DOI] [PubMed] [Google Scholar]
- Gieffers C, Peters BH, Kramer ER, Dotti CG, Peters JM. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci USA. 1999;96:11317–11322. doi: 10.1073/pnas.96.20.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmachl M, Gieffers C, Podtelejnikov AV, Mann M, Peters JM. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc Natl Acad Sci USA. 2000;97:8973–8978. doi: 10.1073/pnas.97.16.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:795–823. [PubMed] [Google Scholar]
- Hatfield PM, Gosink MM, Carpenter TB, Vierstra RD. The ubiquitin-activating enzyme (E1) gene family in Arabidopsis thaliana. Plant J. 1997;11:213–226. doi: 10.1046/j.1365-313x.1997.11020213.x. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ganoth D, Sudakin V, Dahan A, Cohen LH, Luca FC, Ruderman JV, Eytan E. Components of a system that ligates cyclin to ubiquitin and their regulation by the protein kinase cdc2. J Biol Chem. 1994;269:4940–4946. [PubMed] [Google Scholar]
- Ito M. Factors controlling cyclin B expression. Plant Mol Biol. 2000;43:677–690. doi: 10.1023/a:1006336005587. [DOI] [PubMed] [Google Scholar]
- Jiang F, Basavappa R. Crystal structure of the cyclin-specific ubiquitin-conjugating enzyme from clam E2-C at 20 A resolution. Biochemistry. 1999;38:6471–6478. doi: 10.1021/bi9901329. [DOI] [PubMed] [Google Scholar]
- Jones D, Crowe E, Stevens TA, Candido EP. Functional and phylogenetic analysis of the ubiquitylation system in Caenorhabditis elegans: ubiquitin-conjugating enzymes ubiquitin-activating enzymes and ubiquitin-like proteins. Genome Biol. 2001;3:RESEARCH0002.1–0002.15. doi: 10.1186/gb-2001-3-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang YL, Huang J, Peters JM, McLaughlin ME, Tai CY, Pellman D. APC-mediated proteolysis of Ase1 and the morphogenesis of the mitotic spindle. Science. 1997;275:1311–1314. doi: 10.1126/science.275.5304.1311. [DOI] [PubMed] [Google Scholar]
- King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Leverson JD, Joazeiro CA, Page AM, Huang HK, Hieter P, Hunter T. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol Biol Cell. 2000;11:2315–2325. doi: 10.1091/mbc.11.7.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287:2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- McGarry TJ, Kirschner MW. Geminin an inhibitor of DNA replication is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Menand B, Maréchal-Drouard L, Sakamoto W, Dietrich A, Wintz H. A single gene of chloroplast origin codes for mitochondrial and chloroplastic methionyl-tRNA synthetase in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1998;95:11014–11019. doi: 10.1073/pnas.95.18.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuzawa H, Seino H, Yamao F, Ishihama A. Two WD repeat-containing TATA-binding protein-associated factors in fission yeast that suppress defects in the anaphase-promoting complex. J Biol Chem. 2001;276:17117–17124. doi: 10.1074/jbc.M100248200. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Regulation of the APC and the exit from mitosis. Nat Cell Biol. 1999;1:E47–E53. doi: 10.1038/10039. [DOI] [PubMed] [Google Scholar]
- Murray A. Cyclin ubiquitination: the destructive end of mitosis. Cell. 1995;81:149–152. doi: 10.1016/0092-8674(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S. Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol. 1992;132:1–30. [Google Scholar]
- Osaka F, Seino H, Seno T, Yamao F. A ubiquitin-conjugating enzyme in fission yeast that is essential for the onset of anaphase in mitosis. Mol Cell Biol. 1997;17:3388–3397. doi: 10.1128/mcb.17.6.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AM, Hieter P. The anaphase-promoting complex: new subunits and regulators. Annu Rev Biochem. 1999;68:583–609. doi: 10.1146/annurev.biochem.68.1.583. [DOI] [PubMed] [Google Scholar]
- Peters JM. Subunits and substrates of the anaphase-promoting complex. Exp Cell Res. 1999;248:339–349. doi: 10.1006/excr.1999.4443. [DOI] [PubMed] [Google Scholar]
- Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Segers G, Gadisseur I, Bergounioux C, de Almeida Engler J, Jacqmard A, Van Montagu M, Inzé D. The Arabidopsis cyclin-dependent kinase gene cdc2bAt is preferentially expressed during S and G2 phases of the cell cycle. Plant J. 1996;10:601–612. doi: 10.1046/j.1365-313x.1996.10040601.x. [DOI] [PubMed] [Google Scholar]
- Shen WH, Parmentier Y, Hellmann H, Lechner L, Dong A, Masson J, Granier F, Lepiniec L, Estelle M, Genschik P. Null mutation of AtCUL1 causes arrest in early embryogenesis in Arabidopsis. Mol Biol Cell. 2002;13:1916–1928. doi: 10.1091/mbc.E02-02-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A. The cyclosome a large complex containing cyclin-selective ubiquitin ligase activity targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995;6:185–197. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P, Fuchs SY, Chen A, Wu K, Gomez C, Ronai Z, Pan ZQ. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of I kappa B alpha. Mol Cell. 1999;3:527–533. doi: 10.1016/s1097-2765(00)80481-5. [DOI] [PubMed] [Google Scholar]
- Thoma S, Sullivan ML, Vierstra RD. Members of two gene families encoding ubiquitin-conjugating enzymes, AtUBC1-3 and AtUBC4-6, from Arabidopsis thaliana are differentially expressed. Plant Mol Biol. 1996;31:493–505. doi: 10.1007/BF00042223. [DOI] [PubMed] [Google Scholar]
- Tokumoto M, Nagahama Y, Tokumoto T. Molecular cloning of cDNA encoding a cyclin-selective ubiquitin carrier protein (E2-C) from Carassius auratus (goldfish) and expression analysis of the cloned gene. FEBS Lett. 1999;458:375–377. doi: 10.1016/s0014-5793(99)01189-8. [DOI] [PubMed] [Google Scholar]
- Townsley FM, Aristarkhov A, Beck S, Hershko A, Ruderman JV. Dominant-negative cyclin-selective ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase. Proc Natl Acad Sci USA. 1997;94:2362–2367. doi: 10.1073/pnas.94.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley FM, Ruderman JV. Functional analysis of the Saccharomyces cerevisiae UBC11 gene. Yeast. 1998;14:747–757. doi: 10.1002/(SICI)1097-0061(19980615)14:8<747::AID-YEA271>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Valvekens D, Van Montagu M, Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodermaier HC. Cell cycle: waiters serving the destruction machinery. Curr Biol. 2001;11:R834–837. doi: 10.1016/s0960-9822(01)00498-5. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Hatakeyama S, Kominami K, Kitagawa M, Matsumoto M, Nakayama K. Cell cycle-dependent expression of mammalian E2-C regulated by the anaphase-promoting complex/cyclosome. Mol Biol Cell. 2000;11:2821–2831. doi: 10.1091/mbc.11.8.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, King RW, Peters JM, Kirschner MW. Identification of a novel ubiquitin-conjugating enzyme involved in mitotic cyclin degradation. Curr Biol. 1996;6:455–466. doi: 10.1016/s0960-9822(02)00513-4. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]