Abstract
The effects of the ionophore X-537A were studied on carbamylcholine (carbachol)-induced densitization and on tension development in relaxed potassium-depolarized frog sartorius muscles. 2 X-537A accelerated carbachol-induced desensitization in Ca2+-deficient solutions without having any effect on the conductance of the membrane in the absence of carbachol or on the extent of the carbachol-induced increase in conductance. 3 In Ca2+-deficient solution, the acceleration of desensitization by the ionophore was concentration-dependent. No effect was observed with concentrations less than 5 muM and maximal acceleration was evident with 10 muM. 4 The influence of X-537A on desensitization was time-dependent. At 20 muM X-537A, there was a marked acceleration of desensitization by the end of 5 min exposure. An additional gradual acceleration occurred during a 5 to 30 min treatment. No acceleration of desensitization was evident when X-537A was simultaneously applied with carbachol to the end-plate region without prior exposure to the ionophore. 5 Desensitization also was accelerated by 30 min exposure to 20 muM X-537A in solutions containing Ca2+ or deficient in both Mg2+ and Ca2+; the rate being increased 2.8-fold in Ca2+-containing solutions, 2.9-fold in Ca2+-deficient solutions containing Mg2+, and 2.5-fold in divalent cation-deficient solutions. 6 Tension development gradually occurred in relaxed potassium-depolarized muscle preparations exposed to 20 muM X-537A. The onset of tension development occurred only after approximately 25 min of exposure both in preparations kept in Ca2+-deficient or Ca2+-containing solutions. By the end of 90 min in the ionophore, the tension developed was approximately 12% and 23% of the initial potassium contracture in those preparations maintained in the Ca2+-deficient or Ca2+-containing solutions, respectively. 7 We assume that the increase in desensitization rate following exposure to X-537A results from an elevation of the intracellular Ca2+ concentration. That muscle tension gradually increased during exposure to the ionophore supports this conclusion. The acceleration of densitization by X-537A in the absence of external Ca2+ supports the view that the site of calcium acceleration is not on the external surface of the end-plate membrane either at or near the agonist-recognition site but rather on the inner surface.
Full text
PDF


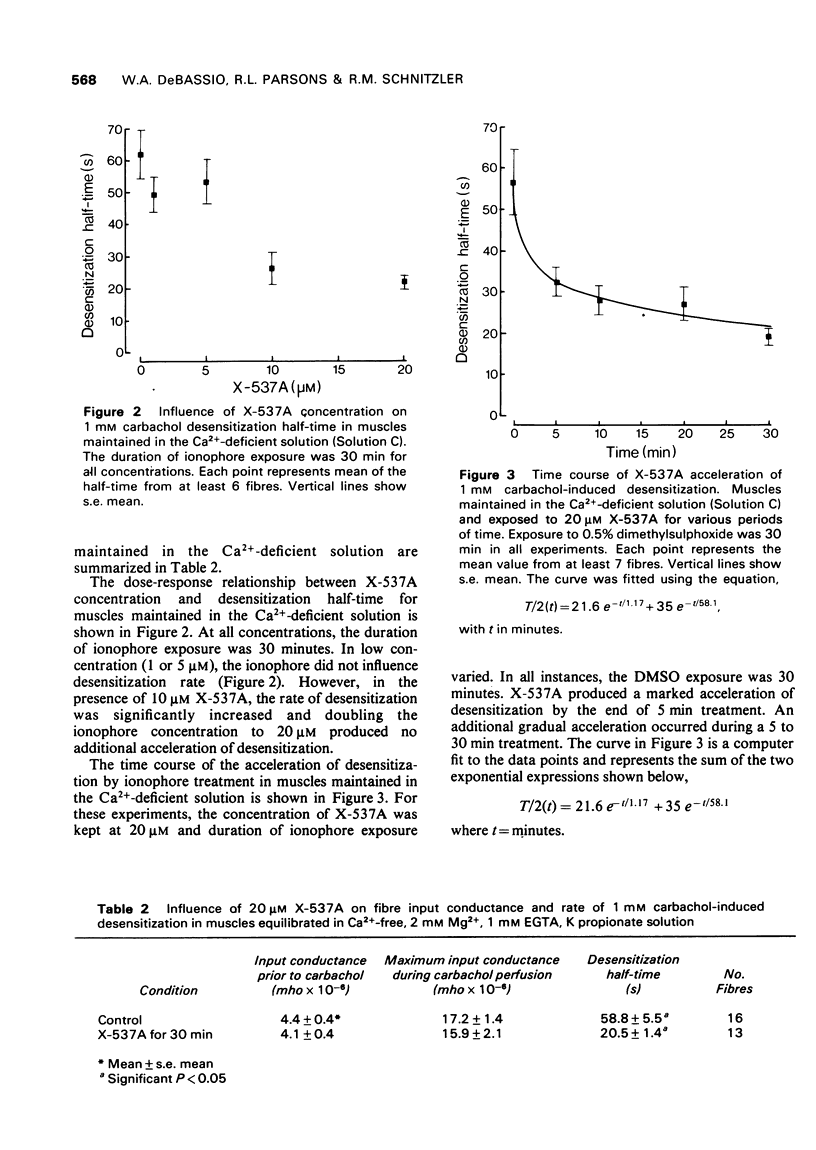



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochrane D. E., Douglas W. W., Mouri T., Nakazato Y. Calcium and stimulus-secretion coupling in the adrenal medulla: contrasting stimulating effects of the ionophores X-537A and A23187 on catecholamine output. J Physiol. 1975 Nov;252(2):363–378. doi: 10.1113/jphysiol.1975.sp011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane D. E., Parsons R. L. The interaction between caffeine and calcium in the desensitization of muscle postjunctional membrane receptors. J Gen Physiol. 1972 Apr;59(4):437–461. doi: 10.1085/jgp.59.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore D. I., Nastuk W. L. Effects of 'calcium ionophore' X537A on frog skeletal muscle. Nature. 1975 Feb 20;253(5493):644–646. doi: 10.1038/253644a0. [DOI] [PubMed] [Google Scholar]
- Entman M. L., Gillette P. C., Wallick E. T., Pressman B. C., Schwartz A. A study of calcium binding and uptake by isolated cardiac sarcoplasmic reticulum: the use of a new ionophore (X537A). Biochem Biophys Res Commun. 1972 Aug 21;48(4):847–853. doi: 10.1016/0006-291x(72)90685-7. [DOI] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. W., Parsons R. L. Characteristics of postjunctional carbamylcholine receptor activation and inhibition. Am J Physiol. 1972 Mar;222(3):793–799. doi: 10.1152/ajplegacy.1972.222.3.793. [DOI] [PubMed] [Google Scholar]
- Kanno T., Cochrane D. E., Douglas W. W. Exocytosis (secretory granule extrusion) induced by injection of calcium into mast cells. Can J Physiol Pharmacol. 1973 Dec;51(12):1001–1004. doi: 10.1139/y73-153. [DOI] [PubMed] [Google Scholar]
- Lambert D. H., Parsons R. L. Influence of polyvalent cations on the activation of muscle end plate receptors. J Gen Physiol. 1970 Sep;56(3):309–321. doi: 10.1085/jgp.56.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapa A. J., Albuquerque E. X., Daly J. An electrophysiological study of the effects of D-tubocurarine, atropine, and alpha-bungarotoxin on the cholinergic receptor in innervated and chronically denervated mammalian skeletal muscles. Exp Neurol. 1974 May;43(2):375–398. doi: 10.1016/0014-4886(74)90179-4. [DOI] [PubMed] [Google Scholar]
- Levy J. V., Cohen J. A., Inesi G. Contractile effects of a calcium ionophore. Nature. 1973 Apr 13;242(5398):461–463. doi: 10.1038/242461a0. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Kun E. Mode of action of the antibiotic X-537A on mitochondrial glutamate oxidation. Biochem Biophys Res Commun. 1973 Feb 5;50(3):820–825. doi: 10.1016/0006-291x(73)91318-1. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. Further studies of the effect of calcium on the time course of action of carbamylcholine at the neuromuscular junction. J Gen Physiol. 1970 Sep;56(3):407–419. doi: 10.1085/jgp.56.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. The antagonistic effects of calcium and potassium on the time course of action of carbamylcholine at the neuromuscular junction. J Membr Biol. 1972;9(4):319–340. [PubMed] [Google Scholar]
- Manthey A. A. The effect of calcium on the desensitization of membrane receptors at the neuromuscular junction. J Gen Physiol. 1966 May;49(5):963–976. doi: 10.1085/jgp.49.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Nastuk W. L., Parsons R. L. Factors in the inactivation of postjunctional membrane receptors of frog skeletal muscle. J Gen Physiol. 1970 Aug;56(2):218–249. doi: 10.1085/jgp.56.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R. L., Johnson E. W., Lambert D. H. Effects of lanthanum and calcium on chronically denervated muscle fibers. Am J Physiol. 1971 Feb;220(2):401–405. doi: 10.1152/ajplegacy.1971.220.2.401. [DOI] [PubMed] [Google Scholar]
- Parsons R. L., Schnitzler R. M., Cochrane D. E. Inhibition of end-plate desensitization by sodium. Am J Physiol. 1974 Jul;227(1):96–100. doi: 10.1152/ajplegacy.1974.227.1.96. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Scarpa A., Baldassare J., Inesi G. The effect of calcium ionophores on fragmented sarcoplasmic reticulum. J Gen Physiol. 1972 Dec;60(6):735–749. doi: 10.1085/jgp.60.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Inesi G. Ionophore mediated equilibration of calcium ion gradients in fragmented-sarcoplasmic reticulum. FEBS Lett. 1972 May 15;22(3):273–276. doi: 10.1016/0014-5793(72)80248-5. [DOI] [PubMed] [Google Scholar]
- THESLEFT S. The mode of neuromuscular block caused by acetylcholine, nicotine, decamethonium and succinylcholine. Acta Physiol Scand. 1955 Oct 27;34(2-3):218–231. doi: 10.1111/j.1748-1716.1955.tb01242.x. [DOI] [PubMed] [Google Scholar]


