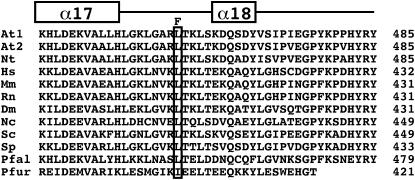
Figure 1.—
The sah1L459F mutation affects a conserved residue in the SAH carboxy-terminal domain. The aligned predicted carboxy-terminal amino acid sequences of SAH from a variety of organisms are shown. These sequences correspond to the region extending from α-helix α17 through α-helix α18 to the carboxy terminus of the protein in the crystal structure of the human SAH protein (Turner et al. 1998), as indicated over the alignment. The residue corresponding to L459 in the Arabidopsis SAH1 protein is boxed, with the mutation to F indicated over this position. The coordinate number of the carboxy-terminal residue for each amino acid sequence is shown in the right margin. At1 is Arabidopsis SAH1, At2 is Arabidopsis SAH2, Nt is Nicotiana tabacum, Hs is Homo sapiens, Mm is Mus musculus, Rn is Rattus norvegicus, Dm is Drosophila melanogaster, Nc is Neurospora crassa, Sc is Saccharomyces cerevisiae, Sp is Schizosaccharomyces pombe, Pfal is Plasmodium falciparum, and Pfur is Pyrococcus furiosus.