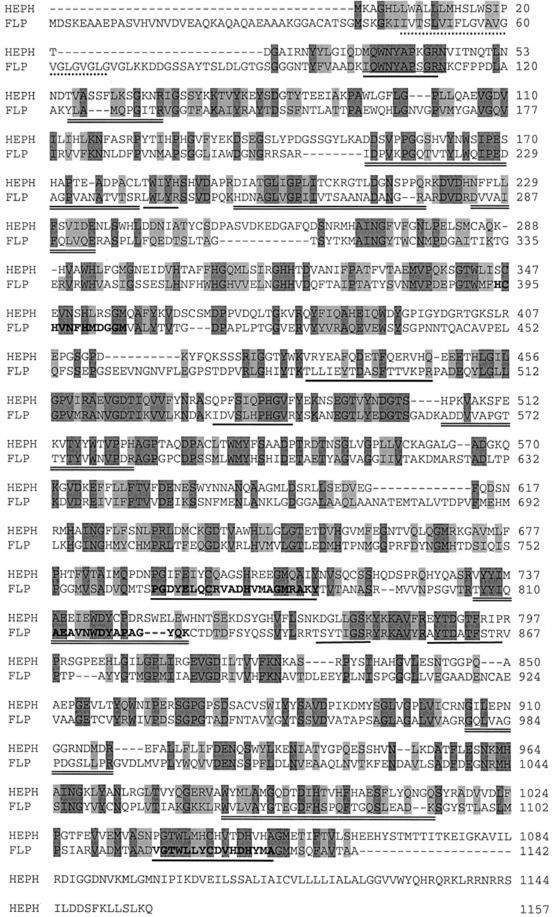
Figure 1.
Comparison of the protein sequence of the FLP from C. reinhardtii with mouse (Mus musculus) HEPH. The alignment was performed using ClustalW from the EMBL database (www.ebi.ac.uk/clustalw). Dark-gray shaded amino acids represent identical residues and light gray indicates conserved substitutions in the alignment. The FLP protein (GenBank accession no. AY074917) and the HEPH protein (GenBank accession no. NP034547) show 31% sequence identity and 46% similarity. Additional features of FLP are also shown. The multicopper oxidase motif I sequences are typed in bold face and underlined, and the multicopper oxidase motif II is typed in bold face. The amino acids 47 to 68 are the putative transmembrane domain and are underlined with a dashed line, the six internal peptide sequences from FLP obtained by MALDI-TOF MS are underlined, and additional peptide maps are underlined twice.