Abstract
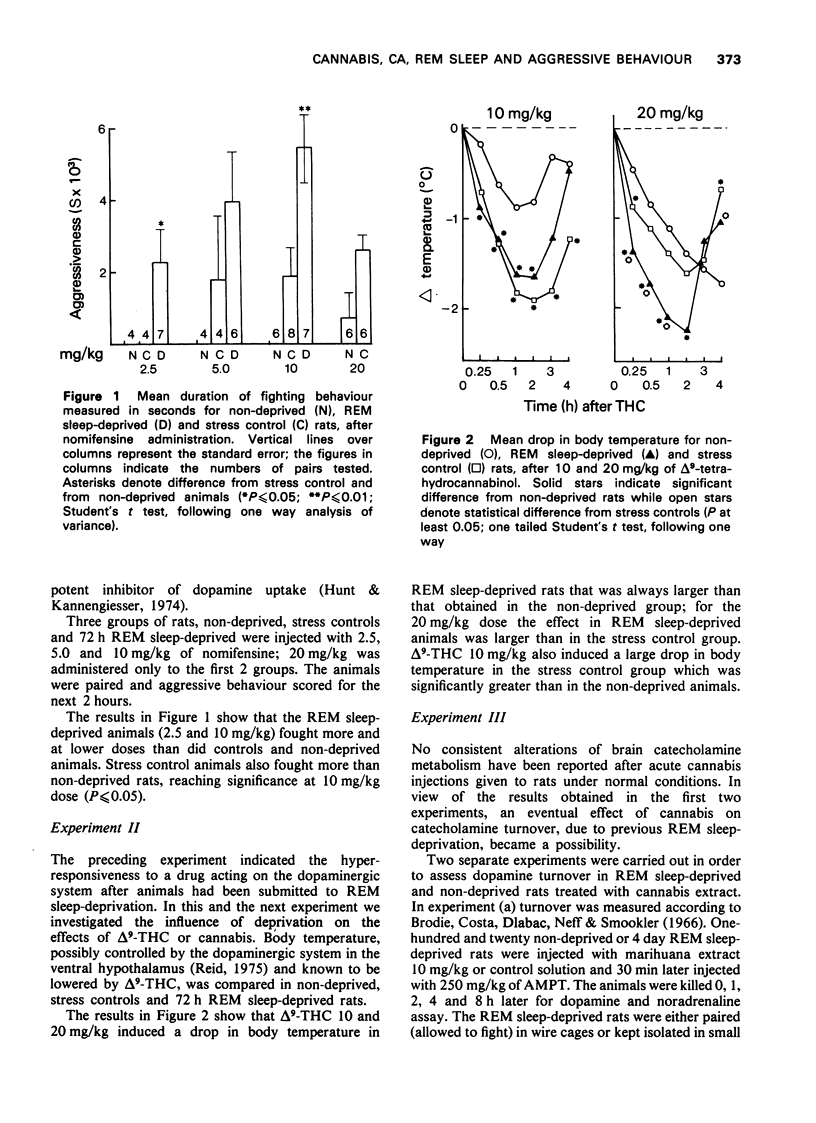
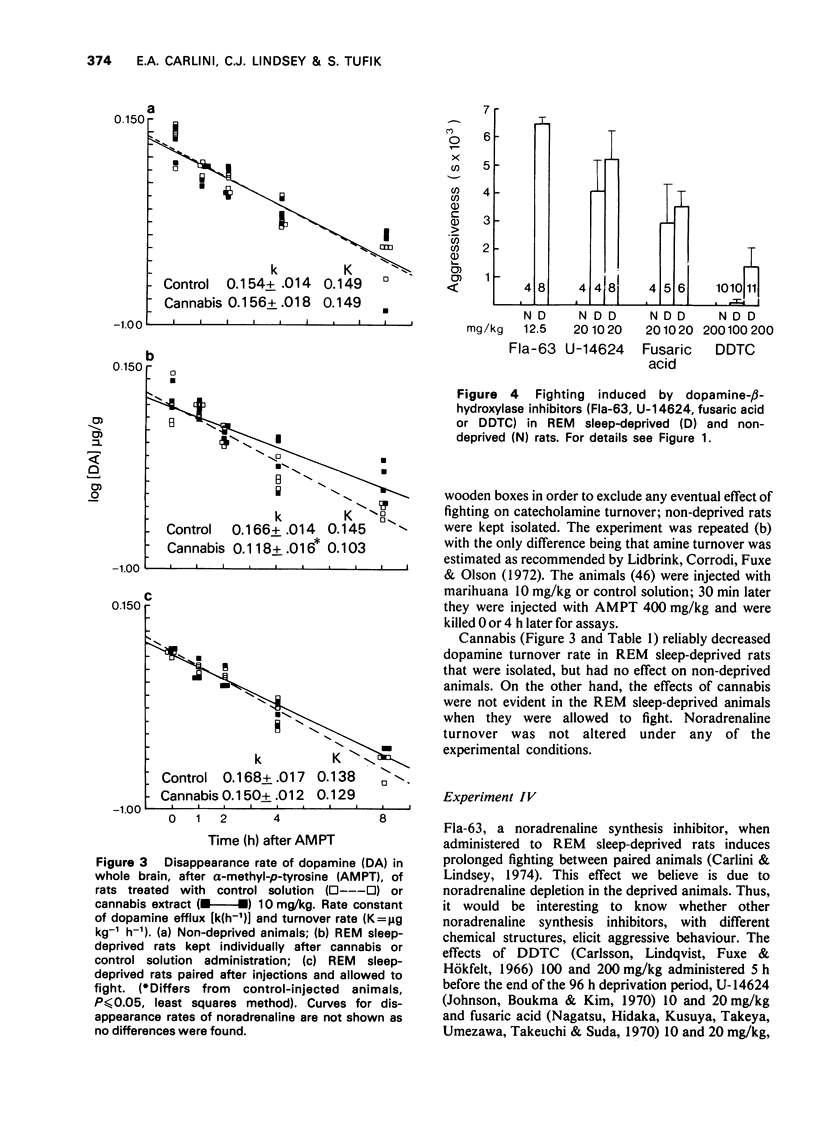
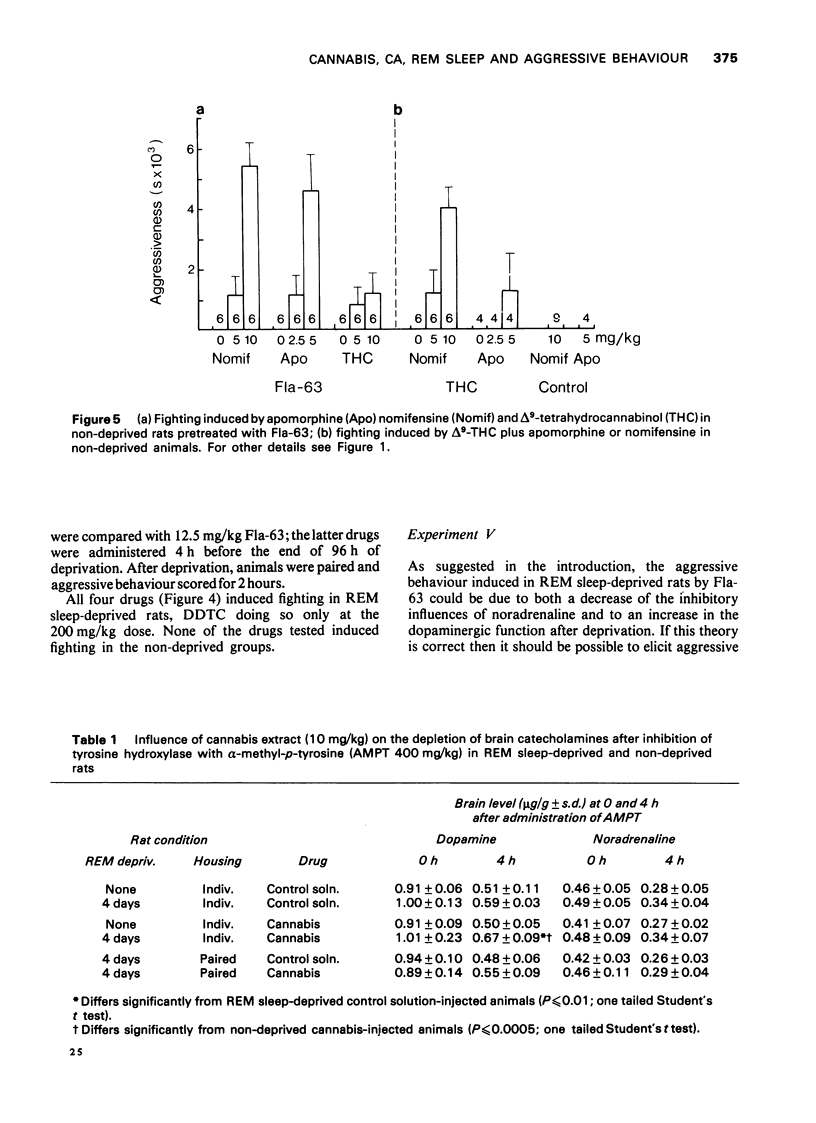
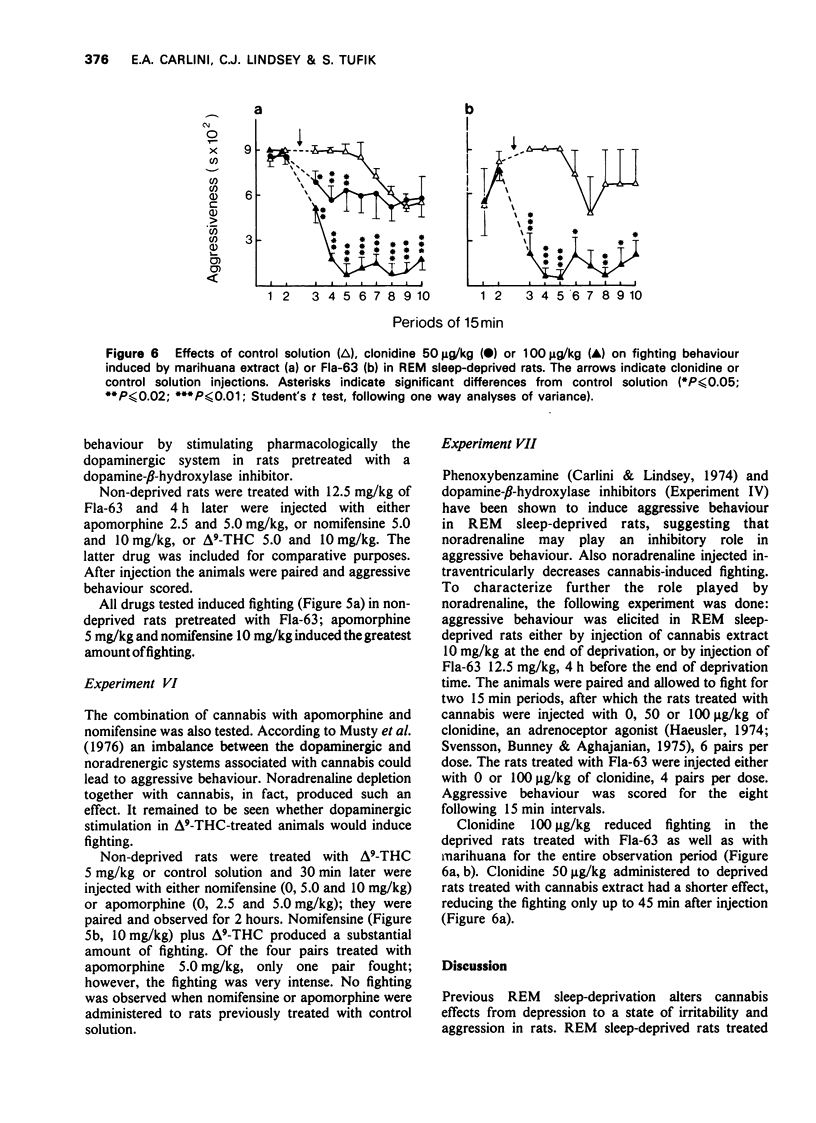
1. Previous work from our laboratory has shown that cannabis induces aggressive behaviour in rats that have been deprived of rapid eye movement (REM) sleep. It was suggested that this effect was related to brain catecholamines, with dopamine playing an agonist role and noradrenaline an inhibitory one. The present paper describes new experiments dealing with this subject. 2. Previous REM sleep-deprivation enhanced both delta9-tetrahydrocannabinol (THC)-induced hypothermia and nomifensine effects on aggressive behaviour. 3. A marihuana extract decreased brain dopamine turnover in REM sleep-deprived rats, an effect not observed in non-deprived rats. Noradrenaline metabolism was not altered. 4. Fighting behaviour was elicited in REM sleep-deprived rats treated with 4 different dopamine-beta-hydroxylase inhibitors. 5. Apomorphine, nomifensine and delta9-THC administered to non-deprived rats pretreated with bis(4-methyl-1-homopiperanzinyl-thiocarbonyl) disulphide (Fla-63), induced fighting behaviour. 6. Nomifensine and apomorphine induced fighting in non-deprived rats pretreated with delta9-THC. 7. Clonidine inhibited the fighting elicited in REM sleep-deprived rats by either delta9-THC or Fla-63 pretreatment. 8. The data are discussed in terms of the influence of REM sleep-deprivation (or the stress associated with deprivation) on the response to dopaminergic drugs and cannabis. Taken together they emphasize the participation of brain dopamine and noradrenaline systems in the aggressive behaviour studied.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- ANTON A. H., SAYRE D. F. THE DISTRIBUTION OF DOPAMINE AND DOPA IN VARIOUS ANIMALS AND A METHOD FOR THEIR DETERMINATION IN DIVERSE BIOLOGICAL MATERIAL. J Pharmacol Exp Ther. 1964 Sep;145:326–336. [PubMed] [Google Scholar]
- Barnes L., Cann F., Karczmar A. G., Kindel G., Longo V. G. Effects of L-dopa on behavior and on brain amines in mice treated with 6-hydroxydopamine. Pharmacol Biochem Behav. 1973 Jan-Feb;1(1):35–40. doi: 10.1016/0091-3057(73)90052-x. [DOI] [PubMed] [Google Scholar]
- Brodie B. B., Costa E., Dlabac A., Neff N. H., Smookler H. H. Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther. 1966 Dec;154(3):493–498. [PubMed] [Google Scholar]
- Carlini E. A., Gonzales C. Aggressive behaviour induced by marihuana compounds and amphetamine in rats previously made dependent on morphine. Experientia. 1972 May 15;28(5):542–544. doi: 10.1007/BF01931867. [DOI] [PubMed] [Google Scholar]
- Carlini E. A., Hamaoui A., Märtz R. M. Factors influencing the aggressiveness elicited by marihuana in food-deprived rats. Br J Pharmacol. 1972 Apr;44(4):794–804. doi: 10.1111/j.1476-5381.1972.tb07317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini E. A., Kramer C. Effects of Cannabis sativa (marihuana) on maze performance of the rat. Psychopharmacologia. 1965 Mar 15;7(3):175–181. doi: 10.1007/BF00411216. [DOI] [PubMed] [Google Scholar]
- Carlini E. A., Lindsey C. J., Tufik S. Environmental and drug interference with effects of marihuana. Ann N Y Acad Sci. 1976;281:229–243. doi: 10.1111/j.1749-6632.1976.tb27934.x. [DOI] [PubMed] [Google Scholar]
- Carlsson A., Lindqvist M., Fuxe K., Hökfelt T. Histochemical amd biochemical effects of diethyldithiocarbamate on tissue catecholamines. J Pharm Pharmacol. 1966 Jan;18(1):60–62. doi: 10.1111/j.2042-7158.1966.tb07773.x. [DOI] [PubMed] [Google Scholar]
- Corrodi H., Fuxe K., Hamberger B., Ljungdahl A. Studies on central and peripheral noradrenaline neurons using a new dopamine-(beta)-hydroxylase inhibitor. Eur J Pharmacol. 1970 Oct;12(2):145–155. doi: 10.1016/0014-2999(70)90059-2. [DOI] [PubMed] [Google Scholar]
- Drew W. G., Miller L. L. Cannabis: neural mechanisms and behavior--a theoretical review. Pharmacology. 1974;11(1):12–32. doi: 10.1159/000136463. [DOI] [PubMed] [Google Scholar]
- Ferguson J., Dement W. The behavioral effects of amphetamine on REM deprived rats. J Psychiatr Res. 1969 Dec;7(2):111–118. doi: 10.1016/0022-3956(69)90016-8. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Sjöqvist F. Hypothermic effect of apomorphine in the mouse. J Pharm Pharmacol. 1972 Sep;24(9):702–705. doi: 10.1111/j.2042-7158.1972.tb09093.x. [DOI] [PubMed] [Google Scholar]
- Geyer M. A., Segal D. S. Shock-induced aggression: opposite effects of intraventricularly infused dopamine and norepinephrine. Behav Biol. 1974 Jan;10(1):99–104. doi: 10.1016/s0091-6773(74)91704-0. [DOI] [PubMed] [Google Scholar]
- Gianutsos G., Hynes M. D., Puri S. K., Drawbaugh R. B., Lal H. Effect of apomorphine and nigrostriatal lesions on aggression and striatal dopamine turnover during morphine withdrawal: evidence for dopaminergic supersensitivity in protracted abstinence. Psychopharmacologia. 1974 Jan 9;34(1):37–44. doi: 10.1007/BF00421218. [DOI] [PubMed] [Google Scholar]
- Goldstein M., Freedman L. S., Backstrom T. The inhibition of catecholamine biosynthesis by apomorphine. J Pharm Pharmacol. 1970 Sep;22(9):715–717. doi: 10.1111/j.2042-7158.1970.tb12763.x. [DOI] [PubMed] [Google Scholar]
- Graham J. D., Lewis M. J., Li D. M. The effect of delta1-tetrahydrocannabinol on the release of (3H-(-)-noradrenaline from the isolated vas deferens of the rat. Br J Pharmacol. 1974 Oct;52(2):233–236. doi: 10.1111/j.1476-5381.1974.tb09705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler G. Clonidine-induced inhibition of sympathetic nerve activity: no indication for a central presynaptic or an indirect sympathomimetic mode of action. Naunyn Schmiedebergs Arch Pharmacol. 1974;286(1):07–111. doi: 10.1007/BF00499107. [DOI] [PubMed] [Google Scholar]
- Hine B., Friedman E., Torrelio M., Gershon S. Tetrahydrocannabinol-attenuated abstinence and induced rotation in morphine-dependent rats: possible involvement of dopamine. Neuropharmacology. 1975 Aug;14(8):607–610. doi: 10.1016/0028-3908(75)90128-8. [DOI] [PubMed] [Google Scholar]
- Hunt P., Kannengiesser M., Raynaud J. Nomifensine: a new potent inhibitor of dopamine uptake into synaptosomes from rat brain corpus striatum. J Pharm Pharmacol. 1974 May;26(5):370–371. doi: 10.1111/j.2042-7158.1974.tb09294.x. [DOI] [PubMed] [Google Scholar]
- Johnson G. A., Boukma S. J., Kim E. G. In vivo inhibition of dopamine beta-hydroxylase by 1-phenyl-3-(2-thiazolyl)-2-thiourea (U-14,624). J Pharmacol Exp Ther. 1970 Jan;171(1):80–87. [PubMed] [Google Scholar]
- Kramer J., Ben-David M. Suppression of prolactin secretion by acute administration of delta9-THC in rats. Proc Soc Exp Biol Med. 1974 Nov;147(2):482–484. doi: 10.3181/00379727-147-38370. [DOI] [PubMed] [Google Scholar]
- Lidbrink P., Corrodi H., Fuxe K., Olson L. Barbiturates and meprobamate: decreases in cathecholamine turnover of central dopamine and noradrenaline neuronal systems and the influence of immobilization stress. Brain Res. 1972 Oct 27;45(2):507–524. doi: 10.1016/0006-8993(72)90479-9. [DOI] [PubMed] [Google Scholar]
- Littleton J. M., Maclean K. I. Proceedings: The effect of delta-tetrahydrocannabinol (delta-thc) on dopamine metabolism in the rat corpus striatum: the influence of environment. Br J Pharmacol. 1974 May;51(1):117P–117P. [PMC free article] [PubMed] [Google Scholar]
- Masur J., Khazan N. Induction by Cannabis sativa (marihuana) of rhythmic spike discharges overriding REM sleep electrocorticogram in the rat. Life Sci I. 1970 Nov 15;9(22):1275–1280. doi: 10.1016/0024-3205(70)90268-7. [DOI] [PubMed] [Google Scholar]
- Modigh K. Effects of isolation and fighting in mice on the rate of synthesis of noradrenaline, dopamine and 5-hydroxytryptamine in the brain. Psychopharmacologia. 1973 Oct 23;33(1):1–17. doi: 10.1007/BF00428790. [DOI] [PubMed] [Google Scholar]
- Modigh K. Effects of social stress on the turnover of brain catecholamines and 5-hydroxytryptamine in mice. Acta Pharmacol Toxicol (Copenh) 1974 Feb;34(2):97–105. doi: 10.1111/j.1600-0773.1974.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Musty R. E., Lindsey C. J., Carlini E. A. 6-Hydroxydopamine and the aggressive behavior induced by marihuana in REM sleep-deprived rats. Psychopharmacology (Berl) 1976 Jul 28;48(2):175–179. doi: 10.1007/BF00423257. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Hidaka H., Kuzuya H., Takeya K., Umezawa H. Inhibition of dopamine beta-hydroxylase by fusaric acid (5-butylpicolinic acid) in vitro and in vivo. Biochem Pharmacol. 1970 Jan;19(1):35–44. doi: 10.1016/0006-2952(70)90327-8. [DOI] [PubMed] [Google Scholar]
- Reid J. L. Dopamine supersensitivity in the hypothalamus? Adv Neurol. 1975;9:73–80. [PubMed] [Google Scholar]
- Scheel-Kruger J., Randrup A. Aggressive bahviour provoked by pargyline in rats pretreated with diethyldithiocarbamate. J Pharm Pharmacol. 1968 Dec;20(12):948–949. doi: 10.1111/j.2042-7158.1968.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Senault B. Amines cérébrales et comportenment d'aggressivité intraspécifique induit par l'apomorphine chez le rat. Psychopharmacologia. 1974 Jan 11;34(2):143–154. doi: 10.1007/BF00421939. [DOI] [PubMed] [Google Scholar]
- Stolk J. M., Conner R. L., Levine S., Barchas J. D. Brain norepinephrine metabolism and shock-induced fighting behavior in rats: differential effects of shock and fighting on the neurochemical response to a common footshock stimulus. J Pharmacol Exp Ther. 1974 Aug;190(2):193–209. [PubMed] [Google Scholar]
- Svensson T. H., Bunney B. S., Aghajanian G. K. Inhibition of both noradrenergic and serotonergic neurons in brain by the alpha-adrenergic agonist clonidine. Brain Res. 1975 Jul 11;92(2):291–306. doi: 10.1016/0006-8993(75)90276-0. [DOI] [PubMed] [Google Scholar]
- Waters D. H., Glick S. D. Asymmetrical effect of delta-9-tetrahydrocannabinol (THC) on striatal dopamine and behavior. Res Commun Chem Pathol Pharmacol. 1973 Jul;6(1):57–63. [PubMed] [Google Scholar]


