Abstract
We have characterized a novel small heat shock protein gene, viscosity 1 (vis1) from tomato (Lycopersicon esculentum) and provide evidence that it plays a role in pectin depolymerization and juice viscosity in ripening fruits. Expression of vis1 is negatively associated with juice viscosity in diverse tomato genotypes. vis1 exhibits DNA polymorphism among tomato genotypes, and the alleles vis1-hta (high-transcript accumulator; accession no. AY128101) and vis1-lta (low transcript accumulator; accession no. AY128102) are associated with thinner and thicker juice, respectively. Segregation of tomato lines heterogeneous for vis1 alleles indicates that vis1 influences pectin depolymerization and juice viscosity in ripening fruits. vis1 is regulated by fruit ripening and high temperature and exhibits a typical heat shock protein chaperone function when expressed in bacterial cells. We propose that VIS1 contributes to physiochemical properties of juice, including pectin depolymerization, by reducing thermal denaturation of depolymerizing enzymes during daytime elevated temperatures.
Ripening of fleshy fruits is a dynamic transitional period that encompasses a myriad of biochemical and physiological changes leading to easily perceivable alterations in fruit texture, firmness, pigmentation, aroma, and sweetness (Tucker, 1993; Grierson and Fray, 1994). Significant progress has been made in characterizing the molecular components of fruit-ripening process, including ethylene biosynthesis and perception, cell wall depolymerization, light signal transduction, and carotenoid accumulation (Giovannoni, 2001). Fruit ripening-related cell wall depolymerization has been investigated in tomato (Lycopersicon esculentum) pericarp to understand the molecular components that regulate the physiochemical properties of cell walls during plant growth and development in general and fruit textural changes in particular (Brownleader et al., 1999). These studies have provided evidence that depolymerization of polyuronides (Huber and O'Donoghue, 1993; Brummell and Labavitch, 1997) and hemicelluloses (Maclachlan and Brady, 1994; Brummell et al., 1999b) and the loss of Gal (Tong and Gross, 1988) are the most prominent changes that occur in cell walls during fruit ripening. Reverse genetics has been used to delineate effects of several cell wall polymer modifying and depolymerizing enzymes that show coordinated increases during the fruit-ripening process (Giovannoni, 2001). Characterization of the effects of polygalacturonase (Giovannoni et al., 1989; Smith et al., 1990; Kramer et al., 1992; Brummell et al., 1997) and pectin methylesterase in transgenic plants over- or underexpressing these enzymes (Tieman et al., 1992; Tieman and Handa, 1994) showed that they play roles in pectin degradation but do not significantly effect pericarp texture. Impaired expression of two tomato β-glucanases by antisense technology suggested that these enzymes affect fruit metabolism but do not change fruit phenotype (Lashbrook et al., 1998; Brummell et al., 1999a). However, repression of expansin (Exp1; Brummell et al., 1999b) and a fruit lipoxygenase (Kausch, 1996) by cosuppression have been reported to reduce fruit softening. In addition, the overexpression of Exp1 resulted in enhanced fruit softening even in mature green fruit by evoking considerable hemicellulose depolymerization in the absence of polyuronide depolymerization, suggesting a role of components other than pectins in fruit softening (Brummell et al., 1999b). Despite these developments, the overall regulation of fruit textural changes, including cell wall depolymerization, remains to be elucidated (Brownleader et al., 1999; Giovannoni, 2001).
Ripening-related depolymerization and solubilization of tomato fruit cell walls are intimately associated with juice viscosity. Tomato varieties with higher levels of water-insoluble solids or higher precipitate weight ratio (Marsh et al., 1980; Takada and Nelson, 1983), both indicators of reduced cell wall depolymerization, show thicker juice as indicated by the Bostwick value and efflux viscosity of the juice. Processing varieties of tomato, bred for increased juice viscosity, contain larger molecular-sized pectin, higher amounts of water-insoluble solids and juice viscosity, and in general firmer texture compared with parental lines (Barrett et al., 1998). The impaired depolymerization of pectins in the genetically engineered fruits with reduced activities of polygalacturonase (Schuch et al., 1991) and pectin methylesterase (Thakur et al., 1996a, 1996b) show increases in juice viscosity. Taken together, these observations indicate that juice viscosity would provide a reasonable estimate of fruit cell wall solubilization.
In the present investigation, we used juice viscosity as an indicator of cell wall solubilization and depolymerization and tested the possibility of isolating novel genes that affect cell wall depolymerization in ripening fruits. Subtractive cloning of transcripts expressed in high- and low-viscosity juice varieties resulted in isolation of several expressed sequence tags (ESTs) that are differentially expressed during fruit ripening in tomatoes varying in viscosity of the processed juice. We report here characterization of one of these genes, designated as viscosity 1 (vis1), whose transcript accumulation is negatively associated with juice viscosity among diverse tomato-breeding lines. We also report characterization of two differentially regulated alleles of vis1 from different varieties of tomato. Segregation analysis of tomato lines heterogeneous for the two vis1 alleles indicates that vis1 expression influences juice viscosity and cell wall depolymerization. Molecular characterization showed that vis1 is a member of small heat shock protein (sHSP) gene family, and temperature and fruit ripening regulate its expression. VIS1 exhibits chaperone activity when expressed in Escherichia coli and protects bacterial proteins from heat denaturation and increases thermotolerance of bacterial cells. We propose that VIS1 influences cell wall depolymerization by protecting enzymatic activities associated with disintegration of fruit components from daily high temperatures under field conditions.
RESULTS
vis1 Is a Member of a Plant sHSP Gene Family
Subtractive cloning using poly(A+) RNAs from tomato genotypes differing in juice viscosity resulted in isolation of an EST that was preferentially expressed in thin juice tomato genotype (see “Materials and Methods” for details). A full-length cDNA, designated vis1, for this EST was isolated from a red-ripe tomato (cv Rutgers) fruit cDNA library (Kausch and Handa, 1995) and characterized. vis1 encodes a protein of 221 amino acids with molecular mass of 25.7 kD. The deduced amino acid sequence of VIS1 showed strong similarity with members of the sHSP gene family (Fig. 1A). VIS1 contains the sHSP consensus I region, P···GVL motif, a signature typical of sHSPs (Waters, 1995). The sHSP consensus II region is also conserved in VIS1. Pair wise sequence identity of VIS1 with the tomato cytoplasmic class II, cytoplasmic class I, mitochondrial, and chloroplastic sHSPs is 32%, 34%, 35%, and 38%, respectively.
Figure 1.
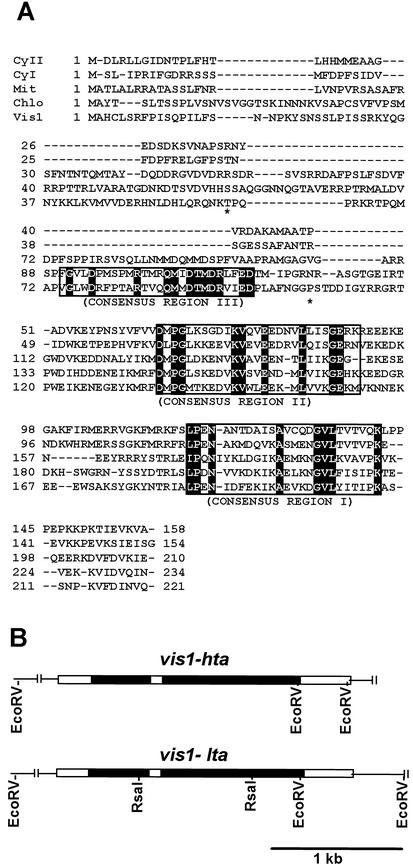
Alignment of the deduced amino acid sequences of VIS1 with other tomato sHSPs (A) and genomic structures of vis1-hta and vis1-lta (B). A, Multiple sequence alignment was performed with ClustalX (Thompson et al., 1997) and manually edited. The three consensus regions in sHSPs are boxed, and identical amino acid residues are highlighted. The asterisks indicate the two residues, Thr61 and Pro107 in VIS1-HTA, that are replaced with Ala in VIS1-LTA. Dashes indicate gaps inserted to improve the alignment. Shown sHSPs are the CyI (cytoplasmic class I; accession no. CAA39603), CyII (cytoplasmic class II; accession no. AAC14577), Mit (mitochondrial; accession no. BAA32547), Chlo (chloroplastic pTOM111; accession no. AAB49626), and Vis1 (VIS1-HTA, accession no. AY128101). B, Shown are the two additional RsaI sites, one in each intron of vis1-lta (accession no. AY128102) but absent in vis1-hta (accession no. AY128101), that were used for developing a PCR-based assay for each allele. The black and white boxes represent introns and exons, respectively.
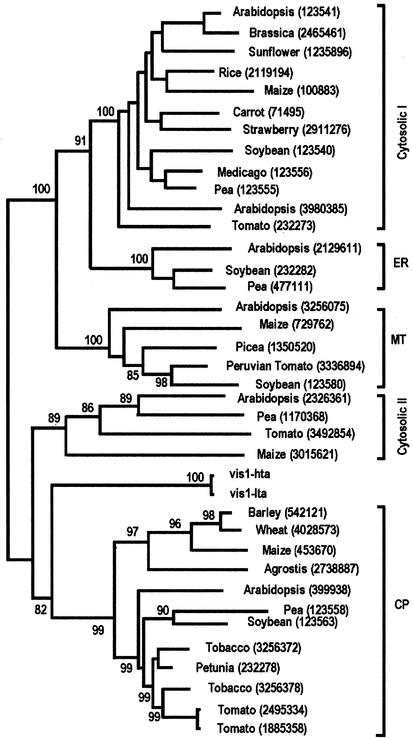
As shown in Figure 2, a phylogenetic tree, using neighbor joining analysis, separated the plant sHSP gene family into five classes with high bootstrap values, indicating the robustness of the tree. VIS1 is closer to chloroplast sHSP than to other sHSP classes. Chloroplast sHSPs share a highly conserved unique consensus III domain containing 28 amino acid residues, of which 18 are identical (Chen and Vierling, 1991). In this region, VIS1 contains only 12 identical amino acid residues. Two protein-targeting prediction programs were inconclusive as to the protein localization. PSORT (Nakai and Kanehisa, 1992) predicts VIS1 to be chloroplast stroma localized, whereas ChloroP (Emanuelsson et al., 1999) predicts VIS1 not to be chloroplast localized. Taken together, these results show that vis1 is a member of plant sHSP gene family.
Figure 2.
Phylogenetic tree of VIS1 and other small sHSPs. Phylogenetic tree is based on deduced amino acid sequences and constructed using neighbor joining analysis as implemented in the Molecular Evolutionary Genetic Analysis package. Bootstrap values are shown at the branch lengths. Numbers in parentheses indicate the accession numbers.
vis1 Expression Is Correlated with Juice Viscosity and DNA Polymorphism
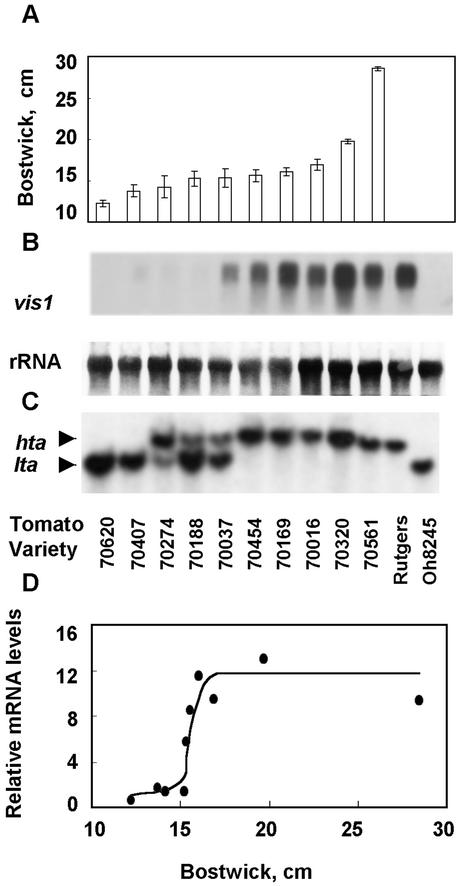
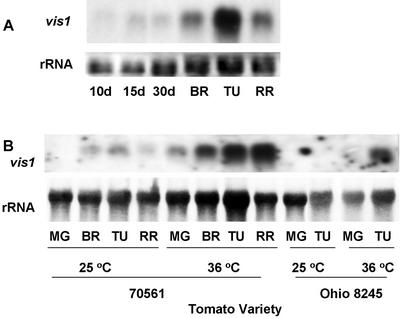
As shown in Figure 3 an apparent relationship was observed between the levels of vis1 transcripts in ripening fruits from diverse genetic backgrounds and viscosity of processed juice. vis1 transcripts were barely detectable in 70620 and 70407, the two lines with the highest juice viscosity (lowest Bostwick value), but increased in lines with lower viscosity (Fig. 3A). However, the relationship between juice Bostwick value and vis1 transcript level is not linear. In general, much higher levels of vis1 transcripts accumulated in genotypes having Bostwick value greater than 15 cm (Fig. 3D). High levels of vis1 transcripts accumulated in fruits of tomato cv Rutgers, a variety with poor processing attributes, whereas vis1 transcripts were not detectable in fruits of tomato cv Ohio 8245, a variety with desirable processing attributes (Berry et al., 1991; Fig. 3B).
Figure 3.
Relationship between Bostwick values, vis1 transcript accumulation, and DNA polymorphism for the vis1-hta and vis1-lta alleles. A, Bostwick values were from the microwave oven-processed tomato juice of fruits grown at the Heinz's research farm (Stockton, CA). Tomato cvs Rutgers and Ohio 8245 were grown at the Purdue research farm and fruits were processed the same way as in California. Bostwick value for the juice processes from tomato cv Ohio 8245 was 15 ± 0.3 cm, whereas juice from tomato cv Rutgers fruits was too thin (Bostwick value >23 cm) to be measured by the standard Bostwick consistometer. B, Equal amounts of total RNAs from turning stages fruit of each line were size fractionated on an agarose gel, blotted to Hybond-N nylon membranes, and hybridized with α-32P labeled vis1 cDNA as described in “Materials and Methods.” Also shown are the levels of 25S RNA. C, Ten micrograms of the genomic DNA from each tomato genotype was digested with EcoRI, separated on an agarose gel, blotted to a Hybond-N membrane, and hybridized with radiolabeled vis1 cDNA. The upper (12-kb) and lower (10-kb) hybridizing bands are designated as vis1-hta and vis1-lta alleles, respectively. D, Relationship between relative vis1 transcript accumulations and Bostwick values for different tomato genotypes. Relative transcript accumulation in fruits from different genotypes was quantified using InstantImager Electric Autoradiography (Packard Instrumental Company, Meriden, CO) and normalized for the amount of ribosomal RNA present in each sample.
Southern-blot analysis of genomic DNA found polymorphism for vis1 among different tomato genotypes that correlated with vis1 transcript accumulation and juice viscosity (Fig. 3C). A single EcoRI-digested genomic DNA fragment from both the lowest and highest juice viscosity genotypes hybridized with vis1. However, as shown in Figure 3C, the size of the hybridizing DNA fragment from the lower juice viscosity genotypes (about 12 kb) was about 2 kb larger than that from the higher juice viscosity genotypes (about 10 kb). The 12-kb fragment associated with high vis1 transcript accumulation was designated as vis1-hta (high-transcript accumulator), and the 10-kb DNA fragment associated with low to undetectable vis1 transcript accumulation was designated as vis1-lta (low transcript accumulator). Several genotypes with intermediate juice viscosity contained both vis1-hybridizing DNA fragments (Fig. 3C) and showed moderate vis1 transcript accumulation. Progeny tests of these lines indicated that they were still heterogeneous for the vis1 polymorphism.
We have cloned and characterized the vis1-hybridizing DNA fragments from 70620 (vis1-lta) and 70320 (vis1-hta) to examine the basis of differential accumulation of vis1 transcripts in these genotypes. The overall organization of vis1 from lower and higher viscosity genotypes is similar and contains three exons interrupted by two introns in the same positions (Fig. 1B). The first intron is of the same size (487 bp) in both vis1-hta and vis1-lta alleles, whereas the size of the second intron differs. The second intron is 1,105 bp in vis1-hta compared with 1,072 bp in vis1-lta. The predicted sizes of three exons are the same, but with two amino acid residue changes, Thr-61 and Pro-107 in VIS1-HTA replaced by Ala-61 and Ala-107 in VIS1-LTA (Fig. 1A). In addition to a 33-nucleotide insertion in vis1-hta, a large number of substitutions are present within the introns of the two alleles (10 in the first and 45 in the second intron, respectively). The vis1-hta and vis1-lta alleles can be identified by the size of RsaI-digested PCR products of the genomic region spanning the second intron because one of the RsaI sites present in the vis1-lta is missing in the vis1-hta (Fig. 1B). Taken together, these results show that the absence of vis1 expression in high juice viscosity tomato genotypes is not attributable to presence of an early stop codon in the vis1-coding region.
vis1-hta and vis1-lta Alleles Segregate with Low and High Juice Viscosity Phenotypes
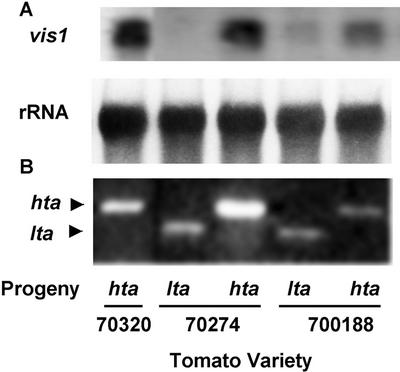
Progenies of two heterozygous lines 70188, and 70274 (Fig. 3C) were used to test the effects of vis1-hta and vis1-lta alleles on juice viscosity (Table I). The benefit of such populations would be that during the inbreeding process, the vis1 allele was carried along in the heterozygous state and much of the background genome would be similar among individuals within each population. The progenies were characterized for the presence of vis1 allele and fruit juice viscosity. For both lines, the vis1-hta and vis1-lta alleles segregated into homozygous and heterozygous genotypes, indicating that they do in fact represent two alleles at the same locus of tomato genome and are not maternally inherited. As shown in Figure 4, the levels of vis1 transcript accumulation were associated with the type of vis1 allele. For all genotypes examined, the progenies with the vis1-hta allele showed higher vis1 transcript accumulation compared with progenies with the vis1-lta allele. Significant increase in juice Bostwick values were observed in progenies with vis1-hta allele compared with progenies with vis1-lta allele for 70188 over 2 years of testing, and even when grown in different locations (Table I). A similar pattern was obtained in line 70274.
Table I.
Effect of vis1-hta and vis1-lta alleles on juice Bostwick value
| Line | Genotype | n* | Bostwick
Value
|
||
|---|---|---|---|---|---|
| Year 1 | Year 2
|
||||
| Site 1 | Site 2 | ||||
| cm 30 s−1 | |||||
| 70188 | hta | 7 | 17.2 ± 1.0 | 18.5 ± 1.5 | 18.3 ± 1.5 |
| 70188 | lta | 5 | 15.7 ± 1.3 | 16.2 + 1.1 | 16.4 + 1.2 |
| P value | 0.038 | 0.009 | 0.023 | ||
| 70274 | hta | 5 | 16.3 ± 1.6 | 14.9 ± 0.7 | 16.1 ± 0.9 |
| 70274 | lta | 1 | 12.1 | 14.2 | 13.5 |
Same segregating progenies from F6 lines were tested for each trial; Bostwick values were presented as mean ± sd. Field trials for year 1 and year 2, site 1 were conducted at Heinz's research farm (Stockton, CA) and for year 2, site 2 at Huron, CA. P values were based on one-tailed distribution of two samples with unequal variance using Student's t test. *n, Number of independent segregates evaluated for each genotype in every trial. Among 70274 progenies tested, one had vis1-lta genotype.
Figure 4.
DNA polymorphism for vis1 is correlated with its transcript accumulation (A) in segregating progenies of tomato genotypes with vis1-hta or vis1-lta allele (B). A, Ripened fruits from each progeny obtained after selfing of tomato genotypes 70188 and 70274 (heterozygous for vis1-hta and vis1-lta alleles) were processed and Bostwick values were determined. Juice Bostwick values of vis1-hta and vis1-lta genotypes of lines 70274 and 70188 were significantly different at the 95% confidence level and correlated with vis1 expression. B, Identity of each segregating progeny, homozygous for vis1-hta or vis1-lta or heterozygous for the two alleles, was established by PCR of their individual genomic DNA followed by digestion with RsaI as described in “Materials and Methods.”
Analysis of Fruit Pectins in vis1-hta and vis1-lta Genotypes
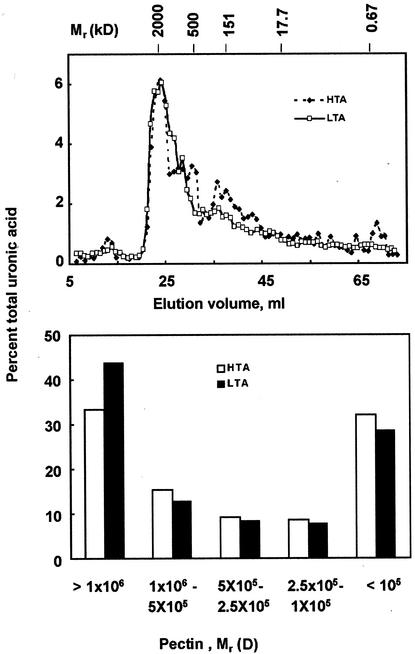
To evaluate the biochemical basis of observed viscosities of vis1-lta and vis1-hta genotypes, we examined the depolymerization of cell wall pectins in ripened fruits from two of the segregating populations. Fruits were harvested 7 d after breaker, and lycopene level was used as an additional criterion to select pericarp representing similar physiological stage of ripening. Total trans-1, 2-cyclohexanediamine-N,N,N′,N′-tetraacetic acid (CDTA)-soluble pectins were extracted from pericarp of vis1-lta and vis1-hta segregants of 70188 and fractionated on a Sepharose CL-4B column. For both vis1-lta and vis1-hta, the major peak of CDTA-extractable polyuronides co-eluted with the blue dextran standard averaging about 2,000 kD in size (Fig. 5A). Ripe fruits from vis1-lta genotype contained higher amounts of pectins larger than 1,000 kD than ripe fruits from vis1-hta genotype (Fig. 5B). The vis1-lta fruits contained approximately 44% of the total CDTA-extractable polyuronic acid with molecular mass greater than 1,000 kD compared with 33% present in the vis1-hta fruits (Fig. 5B). Similar results were obtained from line 70274 (data not shown). These results suggest that reduced depolymerization of polyuronide likely is the basis for thicker viscosity of vis1-lta compared with vis1-hta fruit juice.
Figure 5.
Gel filtration chromatographic analysis of CDTA-soluble polyuronides from red-ripe fruits of segregating progeny of genotype 70188. A, Sepharose CL-4B chromatographic profiles of CDTA-soluble polyuronides isolated from pericarp cell walls from plants segregating for vis1-hta (dashed lines) and vis1-lta alleles (solid lines). Also shown are the elution positions of blue dextran, branched dextrans 17.7 to 500 kD, and bromphenol blue (670 D). B, Distribution of varying sized polyuronides in ripened pericarp of vis1-hta and vis1-lta genotypes. Data represent average of two independent experiments.
Developmental and Temperature Regulation of vis1 Expression
Accumulation of vis1 transcripts was examined in fruit at different stages of development and ripening. vis1 transcripts were barely detectable at early stages of fruit development but rapidly accumulated in fruit after the onset of ripening with maximum accumulation at the turning stage fruit (Fig. 6A). Tomato fruits from vis1-hta and vis1-lta genotypes were treated at 36°C for 6 h to investigate whether expression of vis1 is induced by heat shock. Elevated temperature enhanced the accumulation of vis1 transcripts in both vis1-hta genotypes (70561) and vis1-lta genotypes (tomato cv Ohio 8245; Fig. 6B). The level of pectin methylesterase transcripts remained at similar levels at elevated temperature (data not shown), a result similar to that obtained previously (Kagan Zur et al., 1995).
Figure 6.
vis1 expression is regulated by fruit development, heat treatment, and vis1 alleles. A, Equal amounts of total RNAs from the indicated tissues of tomato cv Rutgers (vis1-hta) were separated on an agarose gel and blotted with the radiolabeled vis1 probe as described in Figure 3. B, Fruits from tomato line 70561 (vis1-hta) and tomato cv Ohio 8245 (vis1-lta) were incubated at the indicated temperature for 6 h, and total RNA was extracted. Other details are as described in Figure 3.
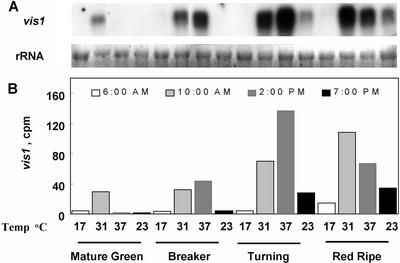
We examined the effects of daily temperature changes on the expression of vis1 in field grown tomatoes. As shown in Figure 7, a transient accumulation of vis1 transcripts was observed as the daytime temperature increased in the field but began to decline after reaching a maximum. The levels of vis1 transcripts correlated with increasing field temperatures and greatly increased with the ripening of fruit (Fig. 7). Taken together, these results indicate that both high temperature and fruit ripening regulate vis1 expression.
Figure 7.
Rhythmic expression of vis1 during daytime temperature changes in field conditions. Tomato cv Rutgers (vis1-hta) fruit at different stages of fruit ripening (MG, mature green; BR, breaker; TU, turning; and RR, red ripe) was collected from field at the indicated times. Shown also are the air temperatures at the time of harvest. Total RNA extraction, northern blotting (A), and quantification of vis1 transcripts (B) were as described in Figure 3.
vis1 Expression Increases Heat Tolerance of Bacterial Cells and Impairs Thermal Denaturation of Bacterial Proteins
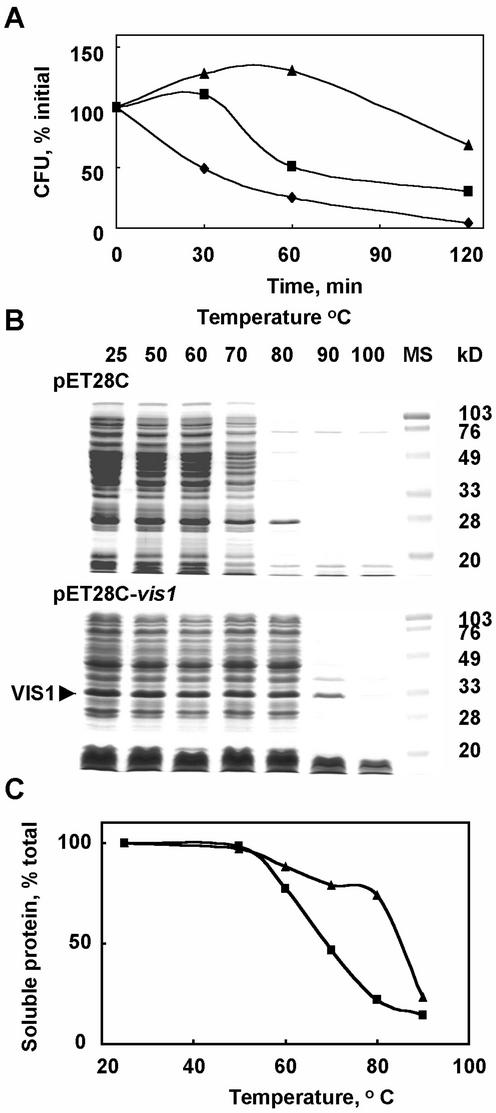
vis1 was expressed in E. coli to characterize its functional attributes including chaperone activity. As shown in Figure 8A, the E. coli BL21 (DE3) cells expressing vis1-hta exhibited enhanced cell viability at 50°C compared with E. coli BL21 (DE3) cells harboring the pET28c vector alone. Induction of vis1 gene expression by IPTG further enhanced the bacterial cell viability. The enhanced cell viability observed in the absence of IPTG is likely attributable to the basal expression of vis1 (pET system manual, Novagen, Madison, WI). At 50°C, bacterial cells expressing vis1 continued to grow during the 1st h, whereas only 25% of the bacterial cells harboring vector alone remained viable (Fig. 8A). After 2 h at 50°C, more than 50% of the vis1-expressing bacterial cells remained viable compared with less than 6% of the cells harboring vector alone. To determine whether VIS1 acts as a chaperone, protein extracts of the bacterial cells with or without vis1 expression were heat-treated at different temperatures. In the absence of VIS1, the bacterial proteins began to aggregate at 60°C with more than 70% protein aggregating at 80°C (Fig. 8C). In the presence of IPTG-induced vis1 expression, the temperature-dependent protein aggregation was highly impaired, and about 80% protein remained soluble after 20 min at 80°C (Fig. 8C). The SDS-PAGE analysis showed that in the presence of VIS1, most proteins remained soluble after a treatment at 80°C for 20 min, whereas only traces of these proteins were soluble in the absence of VIS1 (Fig. 8B). These results demonstrate that VIS1 chaperones proteins against thermal denaturation and enhances viability of bacterial cells at elevated temperatures.
Figure 8.
Expression of vis1 in E. coli increases cell thermo-tolerance (A) and prevents aggregation of the bacterial protein at elevated temperatures (B and C). A, E. coli BL21 (DE3) harboring either the pET28C or pET28C-vis1 were grown at 27°C in Luria-Bertani (LB) plus 50 mg L−1 kanamycin to the initial OD600 of 0.333 (pET28c), 0.205 (pET28c-vis1, without isopropylthio-β-galactoside [IPTG]), and 0.147 (pET28c-vis1, 1 mm IPTG induced for 1 h) and shifted to 50°C. At the indicated time intervals, samples were withdrawn, and the viable cell count was determined using appropriate dilutions on LB plates containing kanamycin after incubation at 37°C overnight. Shown are the viable cell counts for E. coli BL21 (DE3) harboring the pET28C (♦), pET28C-vis1 in the absence of IPTG (▪), and presence of IPTG (▴). B, The bacterial cells harboring either the pET28C or pET28C-vis1 were grown at 27°C in LB medium plus 50 mg L−1 kanamycin in the presence of 1 mm IPTG for 1 h as described above and pelleted by centrifugation (12,000g) for 5 min. Cell pellets were resuspended in a buffer containing 25 mm Tris-HCl, pH 7.5, 10% (v/v) glycerol, 2 mm dithiothreitol, and 1 mm EDTA, sonicated, and centrifuged for 10 min at 12,000g in a microcentrifuge to obtain soluble proteins. Aliquots of soluble protein were incubated at the indicated temperatures for 20 min, and supernatant was collected after centrifugation. Equal volume of supernatants was separated on SDS-PAGE. Shown are the Coomassie R-250-stained gels. Arrow indicates the VIS1 present in the bacterial extracts. C, The percent protein remaining soluble after heat treatment in samples described in B. Symbols are the same as in A. The total soluble protein was determined by the dye-binding assay kit from Bio-Rad (Hercules, CA) using bovine serum albumin as standard.
DISCUSSION
Our approach using the juice thickness as an indicator of fruit cell wall depolymerization led to novel insights into the function of a sHSP in fruit ripening. Among the genes differentially expressed in tomato varieties varying in juice thickness, the expression pattern of one gene, designated as vis1, was negatively correlated with pectin depolymerization and viscosity of the hot-break fruit juice in diverse tomato genotypes (Fig. 3). vis1 shares a high degree of similarity to plant sHSP genes (Figs. 1A and 2) and appears to be closer to the chloroplast sHSP genes than other classes. The location of the first intron in vis1 is identical to the chloroplastic sHSP genes of Arabidopsis and tobacco (Nicotiana tabacum; Osteryoung et al., 1993), but the presence of a unique second intron in vis1 suggests that it is distinct from previously reported chloroplastic sHSP genes. Although many sHSPs have been characterized from plants, molecular functions of only a few have been reported. HSP25 has been linked with heat tolerance in creeping bentgrass (Park et al., 1996), and HSP17.7 affects heat tolerance in carrots (Daucus carota; Malik et al., 1999). The developmentally regulated HSP17.4 is correlated with seed desiccation tolerance in Arabidopsis (Wehmeyer et al., 1996; Wehmeyer and Vierling, 2000). It has been recently proposed that by stabilizing proteins essential for development from environmental stresses, HSP90 plays a significant role against disruptive genetic variations in organisms ranging from insects to plants (Queitsch et al., 2002). In the present study, we provide evidence for a role of a sHSP, vis1, in determining viscosity attributes of tomato fruit juice. To our knowledge, this is the first report of a gene product, other than the enzymatic activities directly involved in depolymerization of fruit cell walls, that regulates physiochemical properties of fruit juice.
Attempts to understand the molecular basis of differential expression of vis1 in thick and thin juice varieties led to characterization of two vis1 alleles. vis1-hta allele is present in thin juice varieties and showed high vis1 transcript accumulation, whereas another allele vis1-lta present in thick juice varieties showed low vis1 transcript accumulation. Although both vis1-hta and vis1-lta encode highly homologous polypeptides differing only in two amino acid residues (Fig. 1A), there were noticeable differences in the nucleotide sequence of the introns present in these alleles (accession nos. AY128101 and AY128102). Some HSP genes have been shown to contain elements in their introns that regulate their expression (Shen et al., 1997; Hirata et al., 1999; Cooper et al., 2000). Whether such regulatory elements are present in the intron regions of vis1-hta and vis1-lta is not known. Our results show that in addition to temperature, fruit ripening regulates accumulation of vis1 transcripts. Although sHSPs are synthesized in response to heat stress and are generally not found in the normal vegetative tissues, the accumulation of some sHSPs has been detected during pollen and embryo development, seed germination, and fruit ripening (Waters et al., 1996; Carranco et al., 1997). A sHSP gene, pTOM111, which shows 38% pair wise sequence amino acid identity to VIS1, is up-regulated during fruit ripening and heat stress (Lawrence et al., 1997). Molecular basis of developmental regulation of HSPs is largely not known. However, there is a possibility that fruit perceives ripening as a stress event and enhances expression of certain stress proteins including VIS1.
Depolymerization of fruit pectins is a common event in the ripening of fleshy fruits. Although polygalacturonase, its β-subunit, and pectin methylesterase have been shown to influence solubilization of fruit pectins (Giovannoni et al., 1989; Smith et al., 1990; Kramer et al., 1992; Tieman et al., 1992; Watson et al., 1994; Brummell et al., 1997), the role of other gene products in this process is not known. Expansin has been shown to be involved in hemicellulose depolymerization, but has little effect on pectin metabolism (Brummell et al., 1999b). We show that fruit expressing higher levels of vis1 (vis1-hta compared with vis1-lta genotypes) contain pectin of relatively smaller size (Fig. 5) and interpret these results as suggesting that vis1 plays a role in pectin depolymerization. Although the molecular role of VIS1 in tomato fruit is not yet clear, the relationship between vis1 expression and juice viscosity can be explained by the following mechanisms. One possibility is that during the daytime rise in temperature, VIS1 acts as chaperone and binds reversibly to enzymes, including cell wall polymer-modifying and -depolymerizing enzymes, and protects them from thermal denaturation. During nighttime, when temperature drops, the VIS1-protected proteins get reactivated and facilitate depolymerization/solubilization of cell walls. However, in the absence of vis1 expression, some of the cell wall-depolymerizing activities undergo irreversible denaturation with rise in the daytime temperature, resulting in a lower rate of cell wall solubilization and thicker juice. The other possibility is that vis1 is linked with another gene(s) that controls juice viscosity.
Ripening in many types of fruits is impaired at elevated temperatures (Paull and Chen, 2000). Tomato fruit kept at a temperature of 30°C and above show abnormal ripening including lack of lycopene accumulation, slowdown in chlorophyll degradation and tissue softening, and decrease in ethylene production (Biggs et al., 1988; Picton and Grierson, 1988). Upon return of heat-stressed fruit to moderate temperatures, ripening recovers, at least partially, but with a delay in the overall ripening process (Biggs et al., 1988; Kagan Zur et al., 1995). Steady-state transcript levels and enzyme activity of several ripening-related genes, including ACC synthase, ACC oxidase, and polygalacturonase, decrease in tomato fruit stored at 35°C (Biggs et al., 1988; Picton and Grierson, 1988; Kagan Zur et al., 1995). We have shown previously that polygalacturonase expression is gradually and irreversibly impaired in fruit at elevated temperatures (Kagan Zur et al., 1995). Symptoms of chilling injuries are reduced after heat treatment, and this reduction is correlated with persistence of several HSPs in fruit tissue (Sabehat et al., 1996). We propose that VIS1, along with other HSPs play a role in facilitating fruit ripening, senescence, and seed dispersal processes by protecting cellular machinery against the thermal denaturation during the daily cycles of daytime rise in temperature.
MATERIALS AND METHODS
Plant Material
Tomato (Lycopersicon esculentum) processing lines, including the segregating progenies of lines 70188 and 70274, were grown at Heinz's research farm (Stockton, CA or Huron, CA). Fully red-ripe fruits (1.3 kg, about 20 fruits) were processed by cooking whole fruits in a commercial microwave oven, pulping, and finishing to remove seeds and skins (Wolcott et al., 1987). Any water lost during cooking was replaced before pulping. After cooling to room temperature, the juice was evaluated for viscosity, soluble solids, and pH (Thakur et al., 1996b). Bostwick value, representing the spread or flow of non-Newtonian fluids, has been used extensively as an indicator of tomato juice viscosity (Gould, 1992).
Breaker stage fruits from each genotype and leaf tissue from the segregating progenies were shipped to Purdue University by the FedEx Express service for determining vis1 expression patterns and genotype of individual plants. Tomato varieties Rutgers, Ohio 8245, 70561, and 70320 were grown either in the greenhouse or on the research farm at Purdue University using routine cultivation practices as described previously (Biggs et al., 1986; Tieman et al., 1995). To obtain fruit at different developmental and ripening stages, either flowers at full opening or fruits at breaker stage were tagged (Biggs el al., 1986). For evaluating the effects of diurnal changes, fruit at mature green, breaker, turning, and red-ripe stages were collected at the indicated times of the day from the research farm at Purdue, and the air temperatures were recorded. To determine the effects of elevated temperature on vis1 expression, greenhouse-grown fruit from line 70561 and tomato cv Ohio 8245 were harvested at the indicated stages, incubated at 36°C for 6 h, and the pericarp frozen in liquid nitrogen. Pericarp collected in the field was frozen in dry ice, whereas all other plant tissues were frozen immediately in liquid nitrogen and stored at −80°C until extraction. Lycopene levels of frozen pericarp were determined as described previously (Handa et al., 1985).
DNA Extraction and Analysis
DNA was extracted as described by Dellaporta et al. (1983). For Southern blotting, 10 μg of genomic DNA was digested with EcoRI, separated on a 1% (w/v) agarose gel, blotted onto Hybond-N membrane (Amersham Biosciences UK, Little Chalfont, Buckinghamshire, UK), and hybridized with α-32P-labeled vis1-probe at 42°C in 50% (v/v) formamide, 6× SSPE, 0.1% (w/v) SDS, 5× Denhardt's solution, and 100 μg mL−1 herring sperm DNA. The cDNA insert of vis1 was labeled using a random primer labeling kit (DECA Prime II, Ambion, Austin, TX) and radiolabeled probe purified on a Sephadex G-50 column. Hybridized membranes were washed two times for 15 min each in 4× SSPE and 0.1% (w/v) SDS at room temperature followed by a 10 min wash in 4× SSPE and 0.1% (w/v) SDS at 55°C. Then, three 10-min washes in 0.1× SSPE and 0.1% (w/v) SDS at 65°C were performed.
RNA Extraction and Analysis
Total RNAs were extracted according to Biggs et al. (1986). Fifteen micrograms of total RNAs was size fractionated on a 1.2% (w/v) agarose denaturing formaldehyde gel, blotted onto Hybond-N nylon membrane (Amersham Biosciences UK) and hybridized to α-32P-labeled vis1 probe at the same conditions described above. After hybridization, membranes were washed twice for 15 min each in 2× SSC and 0.1% (w/v) SDS at room temperature and then twice for 10 min each in 0.2× SSC and 0.1% (w/v) SDS at 62°C. All experiments were repeated at least two times.
Construction and Screening of cDNA Subtraction Library and Identification of vis1 Gene
Ripened fruits from several independent tomato-breeding lines, inbred five to seven generations, were processed in a microwave oven, and juice was quantified for viscosity. The processed juices from these lines exhibited a broad range of viscosity with Bostwick values ranging from 12.2 to 28.0 cm (Fig. 3A). Two breeding lines, 70320 with a Bostwick value of 19.7 cm (thin viscosity) and 70620 with a Bostwick value of 12.2 cm (thick viscosity), were selected to identify genes differentially expressed in thinner and thicker juice genotypes using subtractive cloning (Diatchenko et al., 1996).
Total RNAs were extracted from turning-stage fruits of 70620 and 70320 according to Biggs et al. (1986). The poly(A+) RNA was purified using PolyA spin mRNA isolation kit (New England Biolabs, Beverly, MA) essentially as described by the manufacturer. cDNA subtraction library was constructed using a PCR Select cDNA subtraction kit (BD Biosciences Clontech, Palo Alto, CA) according to manufacturer's instructions (User Manual PT1117–1). The cDNAs made from 70620 and 70320 fruits were used as driver and tester cDNAs, respectively. The resultant subtracted cDNAs were amplified by PCR as described in the manufacturer's instructions, ligated to a TA cloning vector pCR2.1 (Invitrogen, Carlsbad, CA), and electroporated into Escherichia coli DH5α. Resulting cDNA library was screened using subtracted cDNAs screening kit (CLONTECH User Manual PT3138–1). A number of ESTs that presumably expressed in 70320 were selected. Inserts from the selected ESTs were used as probes for northern blots with total RNAs from 70620 and 70320 fruits to identify genes that were preferentially expressed in the low juice viscosity tomato variety. Transcripts of one of these ESTs, EST 5C5, were undetectable in 70620 (high viscosity) but accumulated in 70320 (low viscosity; Fig. 3B).
Isolation of the Full-Length cDNA for EST 5C5
We have previously generated a cDNA library from poly(A+) mRNA of red-ripe fruit of tomato cv Rutgers in Uni-ZAP λ-vector (Kausch and Handa, 1995). The EST 5C5 insert was labeled with [α-32P]dCTP and was used to screen this library to isolate vis1 full-length cDNA clone according to the user's manual (Stratagene, La Jolla, CA). After three rounds of plaque hybridization, in vivo excision of the positive clones resulted in the putative full-length EST 5C5 clones. Several independent clones were sequenced using Taq DyeDeoxy terminator cycle sequencing reactions on an ABI 377 Prism DNA sequencer (Applied Biosystems, Foster City, CA) at the DNA sequencing facility of Iowa State University. BLAST search (http://www.ncbi.nlm.nih.gov) was performed to locate homologies of EST 5C5 in the GenBank databases.
Isolation of vis1 Genomic Sequences and Genotyping of Segregating Lines
The PCR amplification of vis1 genomic sequences using the N-terminal (5′-CATGGCTCATTGCTTATCAAG-3′) and C-terminal (5′-CATTAATGTCAAACACTTTGGG-3′) primers were unsuccessful because of the presence of two introns. Thus additional primers (5′-CCATCATTTGTTGGACTGTCC-3′ and 5′-GGACAGAGTCATAGAGGATC-3′) representing the internal vis1 cDNA sequences were made and used to amplify vis1 genomic sequences from several low and high juice viscosity tomato varieties. The 25 μL of PCR reaction mixture contained 50 ng of plant genomic DNA, 1.5 μm of each primer, 200 μm of each dNTP, 0.6 unit of Taq DNA polymerase, 50 mm KCl, 10 mm Tris-HCl (pH 8.0), and 1.5 mm MgCl2. DNA amplifications were performed in a thermocycler (PerkinElmer Life Sciences, Boston) with the following profile: (a) 94°C for 1 min for 1 cycle, (b) 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min 30 s for 35 cycles, and (c) 72°C for 5 min for 1 cycle. PCR products were separated on agarose gels, eluted, and cloned into TA cloning vector pCR2.1 (Invitrogen) and transformed into E. coli DH5α by electroporation. Both strands from the PCR amplified DNA fragments were sequenced. Analysis of these DNA sequences revealed the presence of two alleles, one in the low juice viscosity variety and the other in the high juice viscosity variety. Additional PCRs using other primers were performed to establish the final genomic sequences of two vis1 alleles.
Genomic DNA of individual progeny of 70274 and 70188 was extracted as described above. PCR was performed with primers (5′-GGACAGAGTCATAGAGGATC-3′) and (5′-CATTAATGTCAAACACTTTGGG-3′). The RsaI-digested PCR products were run on an agarose gel, the vis1-hta allele was distinguished from vis1-lta by its fast electrophoretic mobility.
Sequence Analysis
Pair wise estimates of sequence identity were performed with the program GAP using the Blossum62 matrix of SeqWeb v1.2 of GCG Wisconsin Package v10.1 (Genetics Computer Group, Madison, WI). Protein targeting was analyzed by using PSORT (http://psort.ims.u-tokyo.ac.jp/) and ChloroP (http://www.cbs.dtu.dk/services/ChloroP) servers.
Sequences used in the phylogenetic analysis were retrieved from GenBank. The deduced amino acid sequences of vis1 and other sHSP genes were aligned using the multiple sequence alignment ClustalW package (Thompson et al., 1994). Molecular Evolutionary Genetic Analysis package (Kumar et al., 1994) was used to construct phylogenetic trees based on a distance matrix using neighbor joining analysis. Bootstrapping (1,000 replicates) was performed to quantify the relative support for branches of the inferred phylogenetic tree.
Pectin Analysis
Acetone-insoluble cell wall material was prepared from frozen fruit pericarp according to Tris-buffered Phenol protocol (Huber and O'Donoghue, 1993). CDTA-extractable polyuronides were extracted from dried acetone-insoluble cell wall as described by Brummell and Labavitch (1997). One milligram of dialyzed CDTA-extractable polyuronides was fractionated on Sepharose CL-4B (60 × 1 cm) using 0.2 m ammonium acetate, pH 5.0, as described previously (Tieman et al., 1992). The column was eluted at a rate of 16 mL h−1 and 0.8-mL fractions were collected. Uronic acid contents were determined by the method of Blumenkrantz and Asboe-Hansen (1973). Blue dextran (2,000 kD), branched dextrans ranging between 17.7 to 500 kD, and bromphenol blue (670 D; Sigma-Aldrich, St. Louis) were used to estimate the molecular mass of pectin in different fractions. Because dextrans may not have the same conformation as pectic polymers, the values shown in Figure 3 are merely an estimation of Mr.
Construction of vis1 Bacterial Expression Vector and Heat Tolerance Assay
The vis1 0.7-kb cDNA insert was excised by EcoRI and XhoI and ligated into the EcoRI/XhoI-digested pET28C, an E. coli expression vector (Invitrogen). The ligated products were transformed into E. coli DH5α by electroporation. The resulting vis1 expression construct was sequenced to establish identity and designated as pET28C-vis1. Plasmids from the pET28C-vis1 and pET28C were isolated and used to transform E. coli BL21 (DE3). The resulting transformants were used to characterize the effects of VIS1 on the bacterial cell viability and thermal denaturation of protein.
ACKNOWLEDGMENTS
We thank Drs. Paul M. Hasegawa and Matthew A. Jenks for the critical review of manuscript, Dr. Mario Morales, Marvin Schott, Ron Ryan, and Brian Ballance for field studies and Ms. Kim Kudla and Tantsiana Datsenka for excellent technical assistance.
Footnotes
This research was supported by the U.S. Department of Agriculture/National Research Initiative (grant no. 94–37304–1110) and by the U.S. Department of Agriculture/North Central Biotechnical Initiative (grant no. 96–34340–2711). This is journal paper no. 16,493 of the Purdue University Agricultural Experiment Station.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.012401.
LITERATURE CITED
- Barrett DM, Garcia E, Wayne JE. Textural modification of processing tomatoes. Crit Rev Food Sci. 1998;38:173–258. doi: 10.1080/10408699891274192. [DOI] [PubMed] [Google Scholar]
- Berry SZ, Gould WA, Wiese KL. Ohio 8245 processing tomato. Hortscience. 1991;26:1093–1093. [Google Scholar]
- Biggs MS, Harriman RW, Handa AK. Changes in gene-expression during tomato fruit ripening. Plant Physiol. 1986;81:395–403. doi: 10.1104/pp.81.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs MS, Woodson WR, Handa AK. Biochemical basis of high-temperature inhibition of ethylene biosynthesis in ripening tomato fruits. Physiol Plant. 1988;72:572–578. [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New methods for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Brownleader MD, Jackson P, Mobasheri A, Pantelides AT, Sumar S, Trevan M, Dey PM. Molecular aspects of cell wall modifications during fruit ripening. Crit Rev Food Sci Nutr. 1999;39:149–164. doi: 10.1080/10408399908500494. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Hall BD, Bennett AB. Antisense suppression of tomato endo-1,4-beta-glucanase Cel2 mRNA ac-accumulation increases the force required to break fruit abscission zones but does not affect fruit softening. Plant Mol Biol. 2000;40:615–622. doi: 10.1023/a:1006269031452. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, Dunsmuir P. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell. 1999b;11:2203–2216. doi: 10.1105/tpc.11.11.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA, Labavitch JM. Effect of antisense suppression of endopolygalacturonase activity on polyuronide molecular weight in ripening tomato fruit in fruit homogenates. Plant Physiol. 1997;115:717–725. doi: 10.1104/pp.115.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranco R, Almoguera C, Jordano J. A plant small heat shock protein gene expressed during zygotic embryogenesis but noninducible by heat stress. J Biol Chem. 1997;272:27470–27475. doi: 10.1074/jbc.272.43.27470. [DOI] [PubMed] [Google Scholar]
- Chen Q, Vierling E. Analysis of conserved domains identifies a unique structural feature of a chloroplast heat shock protein. Mol Gen Genet. 1991;226:425–431. doi: 10.1007/BF00260655. [DOI] [PubMed] [Google Scholar]
- Cooper LF, Uoshima K, Guo ZY. Transcriptional regulation involving the intronic heat shock element of the rat hsp27 gene. Biochem Biophys Acta-Gene Struct Expr. 2000;1490:348–354. doi: 10.1016/s0167-4781(00)00005-1. [DOI] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- Diatchenko L, Lau YFC, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED et al. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, VonHeijne G. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Prot Sci. 1999;8:978–984. doi: 10.1110/ps.8.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni J. Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ, Dellapenna D, Bennett AB, Fischer RL. Expression of a chimeric polygalacturonase gene in transgenic rin(ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell. 1989;1:53–63. doi: 10.1105/tpc.1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould WA. Tomato Production, Processing & Technology. Baltimore: CTI Publications; 1992. pp. 323–343. [Google Scholar]
- Grierson D, Fray R. Control of ripening in transgenic tomatoes. Euphytica. 1994;79:251–263. [Google Scholar]
- Handa AK, Singh NK, Biggs MS. Effect of tunicamycin on in vitroripening of tomato pericarp tissue. Physiol Plant. 1985;63:417–424. [Google Scholar]
- Hirata H, Yamamura I, Yasuda K, Kobayashi A, Tada N, Suzuki M, Hirayoshi K, Hosokawa N, Nagata K. Separate cis-acting DNA elements control cell type- and tissue-specific expression of collagen binding molecular chaperone HSP47. J Biol Chem. 1999;274:35703–35710. doi: 10.1074/jbc.274.50.35703. [DOI] [PubMed] [Google Scholar]
- Huber DJ, O'Donoghue EM. Polyuronides in avocado (Persea americana) and tomato (Lycopersicon esculentum) fruits exhibit markedly different patterns of molecular weight downshifts during ripening. Plant Physiol. 1993;102:473–480. doi: 10.1104/pp.102.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan Zur V, Tieman DM, Marlow SJ, Handa AK. Differential regulation of polygalacturonase and pectin methylesterase gene expression during and after heat stress in ripening tomato (Lycopersicon esculentumMill) fruits. Plant Mol Biol. 1995;29:1101–1110. doi: 10.1007/BF00020455. [DOI] [PubMed] [Google Scholar]
- Kausch KD. Molecular cloning and characterization of lipoxygenase from ripening tomato fruit. PhD thesis. West Lafayette, IN: Purdue University; 1996. [Google Scholar]
- Kausch KD, Handa AK. Molecular-cloning and nucleotide-sequence of a lipoxygenase cDNA from ripening tomato fruit. Plant Physiol. 1995;107:669–670. doi: 10.1104/pp.107.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M, Sanders R, Bolkan H, Waters C, Sheehy RE, Hiatt WR. Postharvest evaluation of transgenic tomatoes with reduced levels of polygalacturonase: processing, firmness and disease resistance. Postharvest Biol Technol. 1992;1:241–255. [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetics analysis software for microcomputers. Comput Appl Biosci. 1994;10:189–191. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- Lashbrook CC, Giovannoni JJ, Hall BD, Fischer RL, Bennett AB. Transgenic analysis of tomato endo-beta-1,4-glucanase gene function: role of cel1 in floral abscission. Plant J. 1998;13:303–310. [Google Scholar]
- Lawrence SD, Cline K, Moore GA. Chromoplast development in ripening tomato fruit: identification of cDNAs for chromoplast-targeted proteins and characterization of a cDNA encoding a plastid-localized low-molecular-weight heat shock protein. Plant Mol Biol. 1997;33:483–492. doi: 10.1023/a:1005785321165. [DOI] [PubMed] [Google Scholar]
- Maclachlan G, Brady C. Endo-1,4-β-glucanase, xyloglucanase, and xyloglucan endo-transglycosylase activities versus potential substrates in ripening tomatoes. Plant Physiol. 1994;105:965–974. doi: 10.1104/pp.105.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik MK, Slovin JP, Hwang CH, Zimmerman JL. Modified expression of a carrot small heat shock protein gene, Hsp17.7, results in increased or decreased thermotolerance. Plant J. 1999;20:89–99. doi: 10.1046/j.1365-313x.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- Marsh GL, Buhlert JE, Leonard SJ. Effect of composition upon Bostwick consistency of tomato concentrate. J Food Sci. 1980;45:703–706. [Google Scholar]
- Nakai K, Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW, Sundberg H, Vierling E. Poly(A) tail length of a heat-shock protein RNA is increased by severe heat-stress, but intron splicing is unaffected. Mol Gen Genet. 1993;239:323–333. doi: 10.1007/BF00276930. [DOI] [PubMed] [Google Scholar]
- Park SY, Shivaji R, Krans JV, Luthe DS. Heat-shock response in heat-tolerant and nontolerant variants of Agrostis palustrisHuds. Plant Physiol. 1996;111:515–524. doi: 10.1104/pp.111.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull RE, Chen NJ. Heat treatment and fruit ripening. Postharvest Biol Technol. 2000;21:21–37. doi: 10.1016/s0925-5214(98)00101-x. [DOI] [PubMed] [Google Scholar]
- Picton S, Grierson D. Inhibition of expression of tomato-ripening genes at high temperature. Plant Cell Environ. 1988;11:265–272. [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- Sabehat A, Weiss D, Lurie S. The correlation between heat-shock protein accumulation and persistence and chilling tolerance in tomato fruit. Plant Physiol. 1996;110:531–537. doi: 10.1104/pp.110.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch W, Kanczler J, Robertson D, Hobson G, Tucker G, Grierson D, Bright S, Bird C. Fruit quality characteristics of transgenic tomato fruit with altered polygalacturonase activity. Hortscience. 1991;26:1517–1520. [Google Scholar]
- Shen YF, Liu JH, Wang XZ, Cheng XK, Wang YL, Wu NH. Essential role of the first intron in the transcription of hsp90 beta gene. FEBS Lett. 1997;413:92–98. doi: 10.1016/s0014-5793(97)00883-1. [DOI] [PubMed] [Google Scholar]
- Smith CJS, Watson CF, Bird CR, Ray J, Schuch W, Grierson D. Expression of a truncated tomato polygalacturonase gene inhibits expression of the endogenous gene in transgenic plants. Mol Gen Genet. 1990;224:477–481. doi: 10.1007/BF00262443. [DOI] [PubMed] [Google Scholar]
- Takada N, Nelson P. New consistency method for tomato products-the precipitate weight ratio. J Food Sci. 1983;48:1460–1462. [Google Scholar]
- Thakur BR, Singh RK, Handa AK. Effects of an antisense pectin methylesterase gene on the chemistry of pectins in tomato (Lycopersicon esculentum) fruit juice. J Agric Food Chem. 1996a;44:628–630. [Google Scholar]
- Thakur BR, Singh RK, Tieman DM, Handa AK. Tomato product quality from transgenic fruits with reduced pectin methylesterase. J Food Sci. 1996b;61:85–87. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. Clustal-W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Handa AK. Reduction in pectin methylesterase activity modifies tissue integrity and cation levels in ripening tomato (Lycopersicon esculentumMill.) fruits. Plant Physiol. 1994;106:429–436. doi: 10.1104/pp.106.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Harriman RW, Ramamohan G, Handa AK. An antisense pectin methylesterase gene alters pectin chemistry and soluble solids in tomato fruit. Plant Cell. 1992;4:667–679. doi: 10.1105/tpc.4.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Kausch KD, Serra DM, Handa AK. Field performance of transgenic tomato with reduced pectin methylesterase activity. J Am Soc Hortic Sci. 1995;120:765–770. [Google Scholar]
- Tong CBS, Gross KC. Glycosyl-linkage composition of tomato fruit cell-wall hemicellulosic fractions during ripening. Physiol Plant. 1988;74:365–370. [Google Scholar]
- Tucker GA. Introduction. In: Seymour GB, Taylor JE, Tucker GA, editors. Biochemistry of Fruit Ripening. London: Chapman & Hall; 1993. pp. 1–52. [Google Scholar]
- Waters ER. Molecular evolution of the small heat-shock proteins in plants. Genetics. 1995;141:785–795. doi: 10.1093/genetics/141.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters ER, Lee GJ, Vierling E. Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot. 1996;47:325–338. [Google Scholar]
- Watson CJ, Zheng LS, DellaPenna D. Reduction of polygalacturonase β subunit expression in transgenic tomato plants affects pectin solubilization and degradation during fruit ripening. Plant Cell. 1994;6:1623–1634. doi: 10.1105/tpc.6.11.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E. Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol. 1996;112:747–757. doi: 10.1104/pp.112.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer N, Vierling E. The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiol. 2000;122:1099–1108. doi: 10.1104/pp.122.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolcott T, Marsh GL, Merson RL. Methods for rapidly evaluating consistency potential of new processing tomato varieties. Acta Hortic. 1987;200:115–124. [Google Scholar]