Fig. 3.
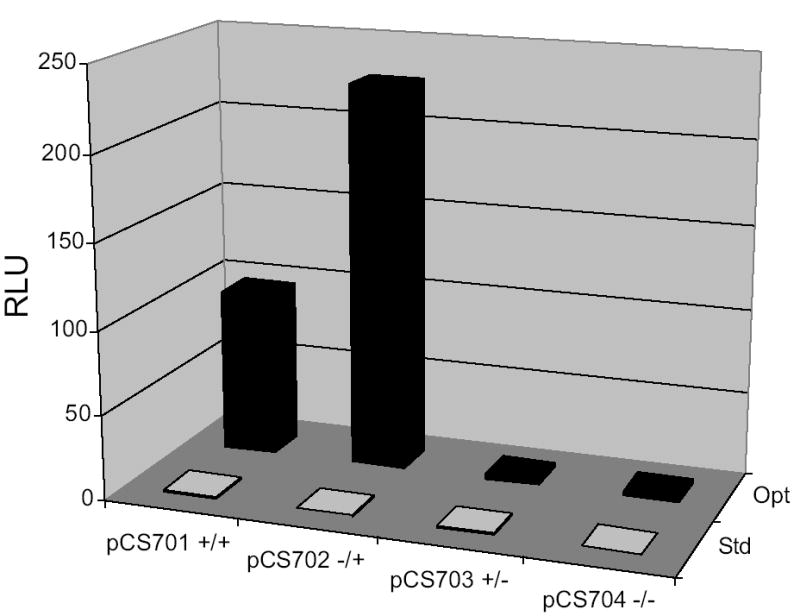
Luciferase activity assays show that translation of mRNAs from pCS701–704 containing the her-2 5′-UTR are highly sensitive to K+ and Mg2+ concentrations in N. crassa extracts. N. crassa in-vitro translation reactions were performed in parallel using 150 mM K+ and 3.75 mM Mg2+ salt concentrations as previously described [11, 15] (denoted Std, standard), or 100 mM K+ and 1.75 mM Mg2+, which yielded optimal synthesis of luciferase from mRNAs containing the her-2 5 leader (denoted Opt). After 30 min of incubation at 25ºC, LUC synthesis was determined by measuring enzymatic activity [expressed as RLU (relative light units)] using 5 μl aliquots from each reaction mixture. The N. crassa optimized salt concentrations showed less than a 2-fold effect on translation of the mRNAs lacking her-2 sequences (T7-LUC) but a 200-fold effect on transcripts containing the her-2 5′-UTR (data not shown). Using optimized salt concentrations, the construct lacking the uORF (pCS702) showed a ~2-fold increase in reporter expression compared to wild-type (pCS701).
