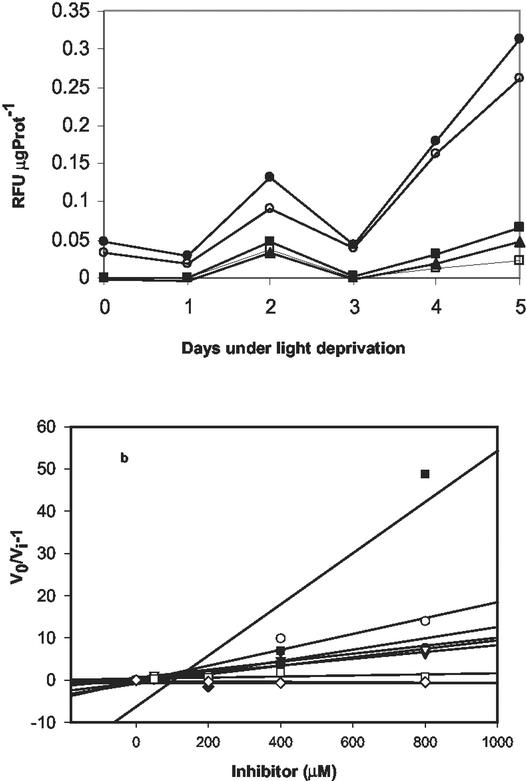
Figure 4.
Caspase activity in D. tertiolecta. Changes in darkness. a, Caspase activity was measured as hydrolysis of 7-amino-4-fluoromethyl coumarin-labeled substrates specific for caspases 1 (WEHD, ▴), 3 (DEVD, □), 6 (VEID, ▪), 8 (IETD, ●), and 9 (LEHD, ○) during the dark period. Symbols are means of triplicate measurements. The difference between the triplicates was less than 10%. RFU μg Prot−1, relative fluorescence units per microgram of protein. b, Inhibition plots of caspase activity in presence of different concentrations of the irreversible broad spectrum inhibitor Boc-D-FMK. v0, Uninhibited rate of reaction; vi, Rate in the presence of an inhibitor. Plotting v0/vi against the concentration of the inhibitor (I) gives a line with a slope equal to 1/dissociation constant of an enzyme-inhibitor complex (Salvesen and Nagase, 1989); thus, greater slopes indicate smaller inhibition constants and greater effects. Activity decreased as the concentration of the inhibitor increased. Complete inhibition was reached at 400 μm inhibitor. Symbols as in a.