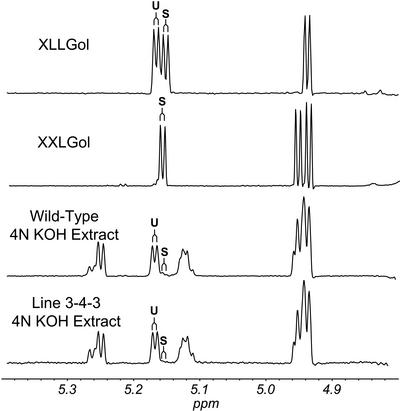
Figure 6.
Diagnostic resonances in the 1H-NMR spectra of XyG oligosaccharides. Example spectra of KOH- or XEG-extracted XyG oligosaccharides from wild-type or atfut1 (which contains a T-DNA insertion in AtFUT1) are shown from 4.2 to 5.3 ppm. All labeled signals are assigned as H1 (anomeric) resonances, except H6 and H6′ of 6-O-acetyl β-D-Galp. The signals labeled α-Glc and β-Glc are assigned to the reducing glucopyranose residue. Anomeric resonances of the two-linked α-D-Xylp residues (labeled 2-Xyl) are also labeled according to their substituent at O2. That is, L indicates a β-D-Gal at O2, F indicates an α-L-Fucp-(1→2)-β-D-Galp moiety at O2, LAc indicates a 6-O-acetyl-β-D-Galp at O2, and FAc indicates an α-L-Fucp-(1→2)-[6-O-acetyl]-β-D-Galp moiety at O2. The anomeric proton resonances of β-D-Galp residues with a α-L-Fucp substituent at O2 (i.e. 2-Gal) are shifted slightly up field (from δ 4.617 to δ 4.606) by O-acetylation at O6, as indicated by the asterisk. The presence or absence of the diagnostic signals, together with the methyl resonance of the O-acetyl substituents (δ 2.13, not shown) at O-6 of β-D-Galp residues, is consistent with the conclusion that fut1 oligosaccharides lack fucosyl residues, and that KOH-extracted oligosaccharides have no O-acetyl substituents.