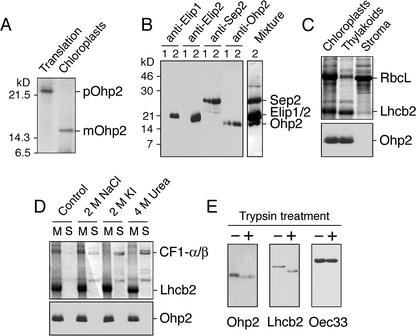
Figure 2.
Location of Ohp2 in thylakoid membranes. A, In vitro import of radioactively labeled Ohp2 into isolated intact pea chloroplasts. pOhp2, Precursor of Ohp2; mOhp2, mature protein. B, Testing of the specificity of polyclonal antibodies raised against overexpressed Ohp2. Leaves were exposed to low-light (lines 1, 100 μmol m–2 s–1) or to light stress (lines 2, 2,500 μmol m–2 s–1) conditions for 3 h, and thylakoid membranes were isolated and used for western blotting with antibodies raised against Elip1, Elip2, Sep2, or Ohp2 from Arabidopsis. For comparison, the western-blot membrane was incubated with a mixture of all four antibodies (Mixture). C, Location of Ohp2 in the thylakoid membrane assayed by immunoblotting. As references, proteins of chloroplast, thylakoid, and stromal fractions were separated by SDS-PAGE, and the distribution of the ribulose-1,5-bisphosphate carboxylase large subunit (RbcL) and the chlorophyll a/b-binding protein of PSII (Lhcb2) was analyzed by Coomassie Blue staining. D, The integral membrane location of Ohp2 was verified by incubation of membranes in the absence (control) or presence of 2 m NaCl, 2 m KI, or 4 m urea. The membrane pellet (lanes M) and supernatant (lanes S) containing extracted peripheral membrane proteins were analyzed by SDS-PAGE followed by Coomassie Blue staining, and the presence of Ohp2 was assayed by immunoblotting. As references, the distribution of the α- and β-subunits of the CF1 ATP synthase complex (CF1-α/β) and the Lhcb2 is shown. E, A protease protection assay was carried out after addition of trypsin (50 μg mL–1) to the isolated thylakoid membranes (1 mg chlorophyll mL–1) and incubation of samples at 4°C for 30 min. The protection of Ohp2 against degradation by trypsin was tested by immunoblotting. As references, the sensitivity of the Lhcb2 toward trypsin treatment and the protection of the lumen located 33-kD protein from the oxygen-evolving complex (Oec33) are shown.