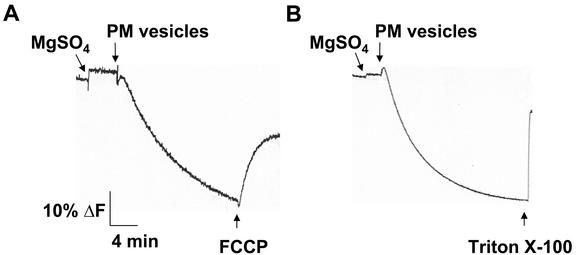
Figure 2.
Characterization of ΔpH formation in aqueous two-phase membrane vesicles isolated from salt-treated Arabidopsis. Plasma membrane vesicles were isolated by two-phase partitioning from leaves of wild-type plants treated with 250 mm NaCl for 3 d. ΔpH was established by the activity of the plasma membrane H+-ATPase and was measured as a decrease (quench) in the fluorescence of the pH-sensitive fluorescent probe quinacrine. Assays (1 mL) contained: 5 μm quinacrine, 3 mm ATP, 100 mm KCl, 25 mm 1,3-bis[tris(hydroxylmethyl) methylamino]propane (BTP)-HEPES (pH 6.5), 250 mm mannitol, and 50 μg of plasma membrane protein. Reactions were illuminated at 430 nm and quinacrine fluorescence was monitored at 500 nm. The protonophore, 5 μm carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP; A) or the detergent 0.02% (w/v) Triton X-100 (B) was added after the ΔpH had reached steady. One representative experiment of three replicates is shown. Each replicate experiment was performed using independent membrane preparations.