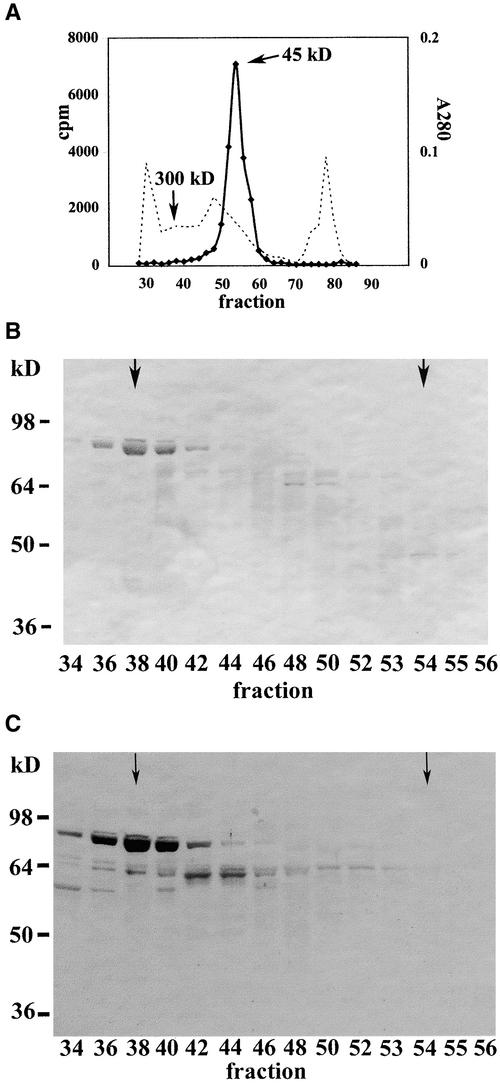
Figure 4.
Immunological Analysis of Partially Purified ZmHda1 after S200 Gel Filtration Chromatography.
(A) The soluble supernatant from a chromatin preparation of 500 g of maize embryos at 72 h after the start of germination was subjected to Q-Sepharose Big Beads chromatography. Those fractions containing ZmHda1 activity without contamination by ZmRpd3 were pooled and subjected to S200 gel filtration chromatography. ZmHda1 enzyme activity eluted in a peak at an apparent molecular weight of ∼45,000. The dashed line depicts the A280 as a rough estimate of the protein concentration.
(B) Fractions from the S200 gel filtration chromatography were blotted onto a nitrocellulose membrane, and proteins were immunodetected with anti-ZmHda1 antibodies (peptide antibodies). Arrows in fractions 38 and 54 mark the molecular weights of 300,000 (ZmHda1-p84) and 45,000 (ZmHda1-p48), respectively.
(C) The same blot shown in (B) was immunoblotted with antibodies against the C-terminal part of ZmHda1. Arrows in fractions 38 and 54 mark the molecular weights of 300,000 (ZmHda1-p84) and 45,000 (ZmHda1-p48), respectively.